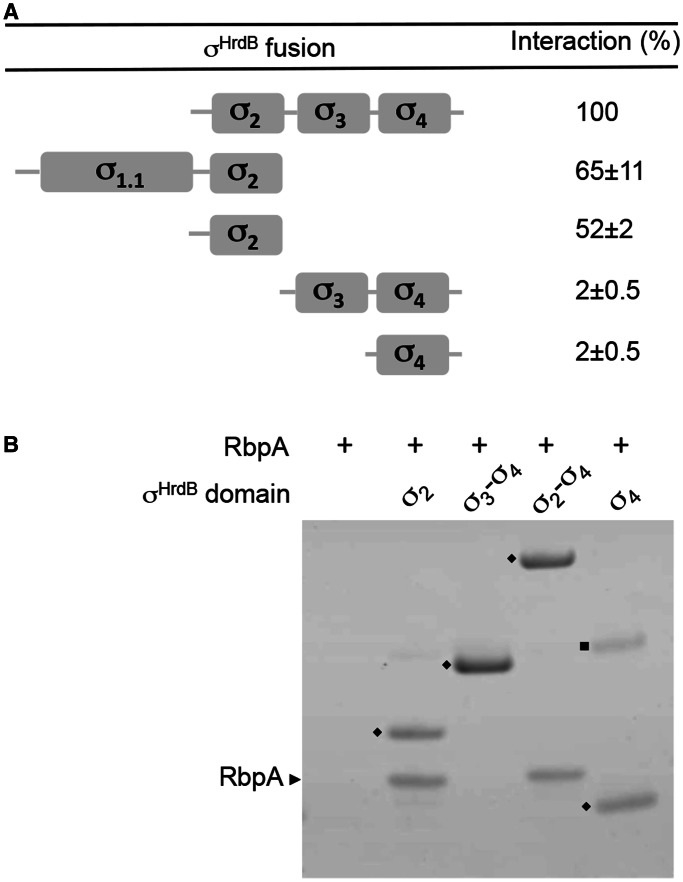
Figure 7.
RbpASc binds to the σ2 domain of σHrdB. (A) A schematic diagram indicating the four conserved regions/globular domains of σHrdB together with BACTH interaction data between truncated T25–σHrdB fusions and RbpASc–T18. The T25–σHrdB fusions included the following amino acids: σ1.1–σ2, 1–347; σ2, 211–347; σ3–σ4, 348–511; σ2–σ4, 211–511; σ4, 435–511. Control strains with pKT25 and pUT18–rbpA exhibited <1% the activity of the σ2–σ4 interaction. Experiments were performed in triplicate, and standard deviations are indicated. (B) Interaction between His6-tagged σHrdB fragments and RbpASc as judged using in vitro pull-down experiments. Purified His6-tagged σHrdB fragments (closed diamond) were mixed with RbpASc (∼0.5–1 μM each) before purification using Ni-affinity magnetic beads. Eluted proteins were separated by 4–12% Bis–Tris SDS–PAGE and stained using Coomassie brilliant blue. RbpASc is indicated with a black arrowhead. Closed square, unknown contaminating protein.