FIGURE 2.
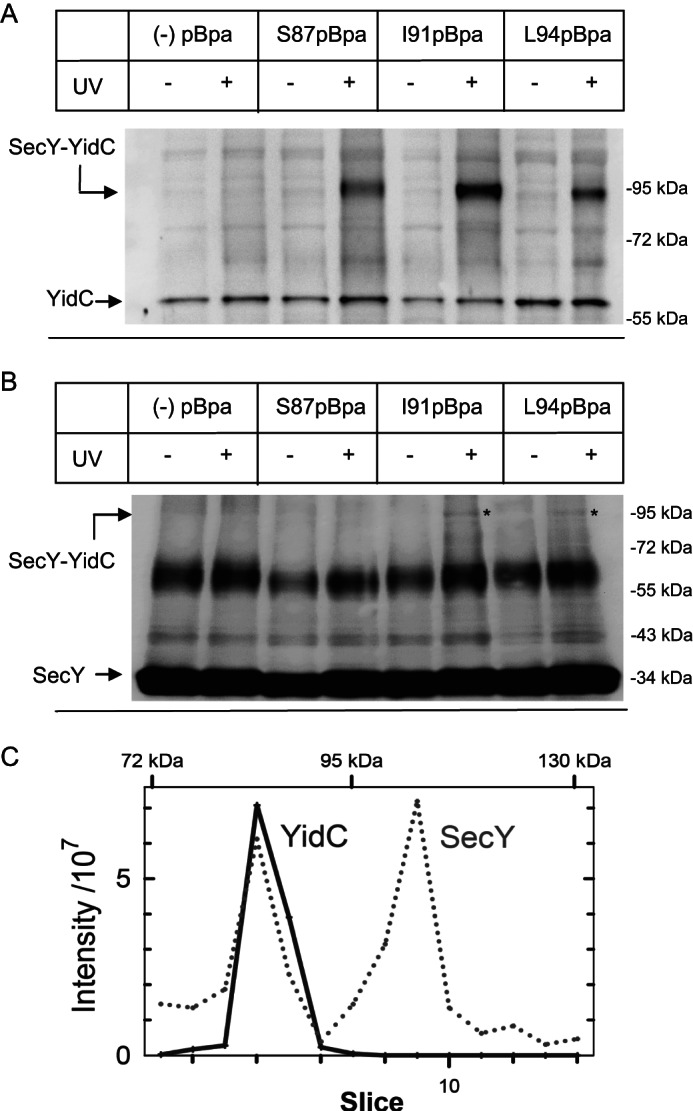
YidC is in contact with helix 2b of the lateral gate of SecY. A, E. coli BL21 cells expressing either wild type SecY (−pBpa) or SecY derivatives carrying pBpa at positions Ser-87, Ile-91, or Leu-94 in TM 2b of SecY were harvested and exposed to UV light for activating pBpa. One aliquot was not UV exposed and served as control. Subsequently, cells were fractionated and SecY/SecY cross-linking products were purified via metal affinity chromatography and separated on SDS-PAGE (approximately 100 μg of protein) followed by immune detection using α-YidC antibodies. Indicated is the SecY-YidC cross-linking product and YidC that co-purified with His-tagged SecY. B, the same material as in A was decorated with α-SecY antibodies, raised against an N-terminal SecY peptide. The putative SecY-YidC cross-linking product is indicated (*). C, a non-UV irradiated (not cross-linked, −UV) and a UV irradiated (cross-linked, +UV) sample of SecY(I91pBpa) purified from whole cells was separated on a 5–15% SDS gel and the proteins were visualized by Coomassie staining. The −UV and +UV lanes were cut into equal slices followed by in-gel trypsin digestion and mass spectrometry. Shown are intensity profiles of SecY (dotted line) and YidC (solid line) peptides found in the individual gel slices. The intensity values were corrected for the values obtained in the absence of UV treatment.
