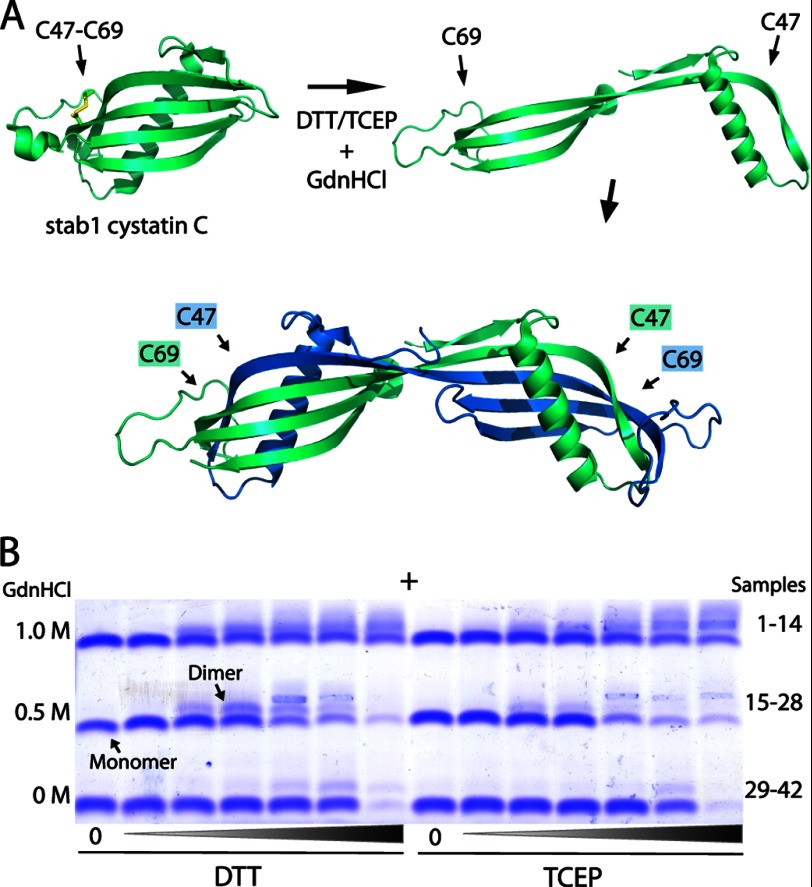
FIGURE 1.
Induced dimer formation of disulfide-stabilized cystatin C. A, the crystal structure of monomeric L47C/G69C (denoted stab1) cystatin C is displayed in a ribbon representation together with partially unfolded and dimeric wt cystatin C, illustrating the mechanism of three-dimensional domain swapping whereby the first hairpin loop (L1) extends and tertiary structure elements are exchanged. The disease-associated L68Q variant shows rapid conversion to dimers under native conditions (17). Under nonreducing conditions stab1 cystatin C is stabilized by the added intramolecular disulfide bond and cannot undergo domain swapping and dimer formation. In a reduction reaction the disulfide is broken, allowing domain swapping and dimerization in mildly denaturing buffers. The figure was prepared in PyMol (43) using coordinates from the structures of Protein Data Bank numbers 3GAX (25) and 1TIJ (44). B, agarose gel electrophoresis of stab1 cystatin C samples, after incubation for 24 h at 40 °C in PBS, pH 7.4, in the absence (samples 1, 8, 15, 22, 29, 36) or presence of the reducing agents DTT or tris(2-carboxyethyl)phosphine (TCEP) (samples 2–7, 16–21, 30–35, and 9–14, 23–28, 37–42, respectively) and low levels of GdnHCl. The optimum yield of conversion to dimers was obtained in 0.5 and 1.0 m GdnHCl by adding exactly 1:1 molar eq of DTT (samples 4 and 18). Higher concentrations of reducing agents resulted in protein precipitation. The anode is marked by a plus sign.