FIGURE 4.
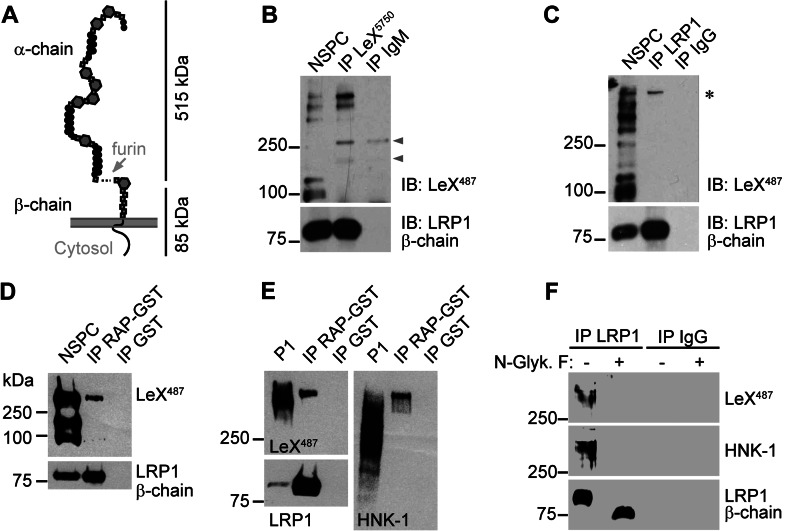
NSPCs express LeX glycoforms of LRP1. A, LRP1 domain structure. Note that LRP1 α- and β-chain are noncovalently linked and run as separate subunits in SDS-PAGE. B, immunoprecipitation (IP) of LeX from NSPCs cultivated as neurospheres. The Western blot against LeX reveals multiple LeX-positive proteins in NSPC input protein lysates. LRP1 precipitates with LeX-positive proteins shown by the detection of LRP1 β-chain. Arrowheads label unspecific antibody signals. C, LRP1 immunoprecipitation from NSPCs yielding a single LeX-positive protein with a size correlating to the expected size of the LRP1 α-chain (asterisk). LRP1 precipitation was confirmed by detection of LRP1 β-chain. D and E, RAP-mediated affinity purification of LRP1 from NSPC (D) or P1 brain (E) lysates. The RAP ligand is positive for LeX and HNK-1. F, Western blots after antibody-mediated immunoprecipitation of LRP1 and subsequent N-glycanase F (N-Glyk. F) treatment. Note that LeX and HNK-1 immunoreactivity on LRP1 α-chain are lost after enzymatic removal of N-linked glycans.