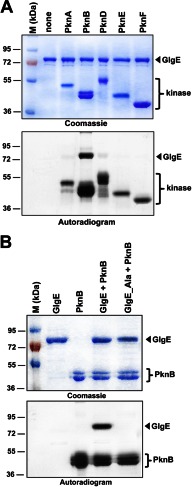
FIGURE 1.
A, in vitro phosphorylation of M. tuberculosis GlgE by PknB. The soluble domains of five recombinant M. tuberculosis STPKs (PknA to PknF) were expressed and purified as His-tagged fusions and incubated with purified His-tagged GlgE and [γ-33P]ATP. The amount of the STPKs used varied from 2 to 4 μg to obtain the optimal autophosphorylation activity for each kinase. Samples were separated by SDS-PAGE, stained with Coomassie Blue (upper panel), and visualized by autoradiography after overnight exposure to a film (lower panel). Upper bands reflect the phosphorylation signal of GlgE, and the lower bands correspond to the autophosphorylation activity of each kinase. M, molecular mass markers. B, in vitro phosphorylation of the GlgE_Ala mutant. Purified GlgE and phosphoablative GlgE (GlgE_Ala) were incubated with PknB and [γ-33P]ATP. Samples were separated by SDS-PAGE, stained with Coomassie Blue (upper panel), and visualized by autoradiography (lower panel) after overnight exposure to a film.