FIGURE 2.
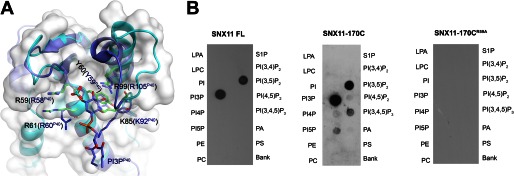
SNX11 interaction with PI3P and PI(3,5)P2. A, superposition of critical residues in the PI-binding pocket of hSNX11-142C and p40phox-PX. hSNX11-142C is shown as a white surface and cyan schematic. The side chains of residues that are critical for PI binding are shown for hSNX11-142C (green) and p40phox-PX (blue). The sulfate in the PI-binding pocket of hSNX11-142C (show as a stick model: orange for sulfur and red for oxygen) coincides with the location of the 3-phosphate of PI3P from p40phox-PX. B, the ability of His-tagged full-length (FL) SNX11, hSNX11-170C, and hSNX11-170C(R59A) to interact with various PIs was determined by lipid overlay assay. LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine 1-phosphate; PI(3,4,5)P3, PI 3,4,5-trisphosphate; PA, phosphatidic acid; PS, phosphatidylserine.
