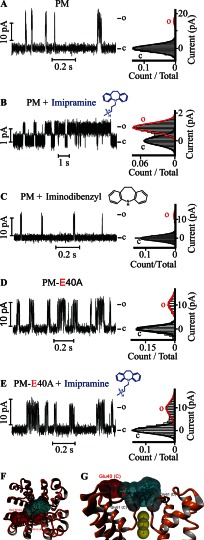
FIGURE 2.
Gating by a charged tricyclic molecule is mediated by a single residue on the PM extracellular surface. Single channel steady-state current records (left) and their corresponding normalized all-point histograms (right) of WT KvLm PM (A and B) and of the KvLm PM E40A mutant (D and E) in symmetric 10% molar DOPA bilayers with 5 μm imipramine in the cis droplet (B and E) and without (A and D) are shown. The structure of imipramine, a tricyclic antidepressant, is shown in blue above the current recordings in B and E. C, single channel currents recorded (left) and the corresponding all-point current histograms (right) from PM reconstituted in symmetric DOPA bilayers in the presence of 5 μm iminodibenzyl on the cis droplet (filter side). The structure of iminodibenzyl, a structural homologue of imipramine lacking the charged amine group, is shown in black. Views from top (F) and side (G) of the highest scoring model for interaction of imipramine with the filter gate of KvLm PM obtained from docking experiments (see “Experimental Procedures”) are shown. The channel PM is depicted in orange ribbons (Protein Data Bank code 4H33). The solvent-accessible surfaces of the interacting residues in the PM (Glu-40, Asp-62, and Gly-61) are shown and are color-coded according to charge (red for Glu-40 and Asp-62 and gray for Gly-61). Yellow arrows show interaction sites (<4 Å). Residues are labeled with subunit in parentheses. The solvent-accessible surface of imipramine is shown and colored light blue. K+ ions at positions S2, S3, and S4 in the filter are shown as solid yellow spheres with their accessible surface depicted. c, closed; o, open.