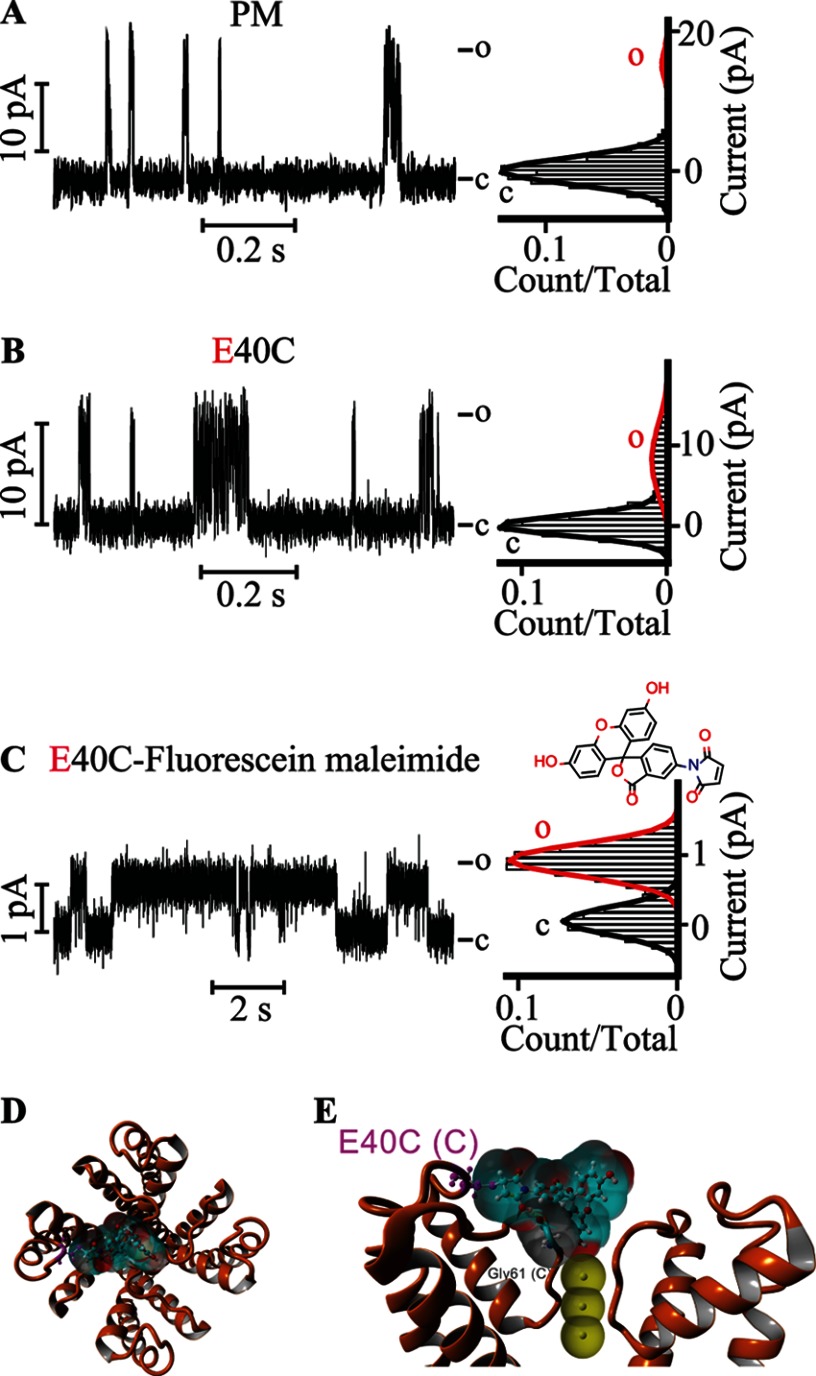
FIGURE 5.
Covalently attached fluorescein at Glu-40 gates the KvLm PM. Single channel steady-state current records (left) and their corresponding normalized all-point histograms (right) of WT KvLm PM (A) and of the KvLm PM E40C mutant (B and C) reconstituted in symmetric DOPA bilayers before (B) and after (C) coupling with fluorescein 5-maleimide are shown. The structure of fluorescein 5-maleimide is shown above the current recording in C. D, extracellular view of a model for interaction of the coupled fluorescein 5-maleimide with the filter gate of KvLm PM (see “Experimental Procedures”). The channel PM is depicted in orange ribbons (Protein Data Bank code 4H33). E, side view of two opposing subunits. The solvent-accessible surfaces of the interacting residues in the PM are shown and are color-coded according to charge (red for Glu-40 and Asp-62 and gray for Gly-61). Yellow dashed lines show hydrogen bond sites. Residues are labeled with subunit in parentheses. The solvent-accessible surface of fluorescein is depicted in light blue. K+ ions at positions S2, S3, and S4 in the filter are depicted as solid yellow spheres displaying their accessible surface. c, closed; o, open.