FIGURE 1.
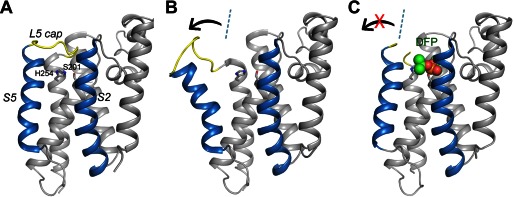
Lateral movement of TM helix S5 in the protease-inhibitor complex is small. A, the apoprotease adopts a closed conformation (Protein Data Bank code 2IC8) (15). The S2 and S5 helices are highlighted in blue. The L5 cap is highlighted in yellow. The catalytic residues are represented by stick models. B, in the open conformation (Protein Data Bank code 2NRF) (16), the C-terminal end of the S5 helix rotate way from the main body of the protease (arrow). The dashed line indicates the orientation of S5 in the closed conformation. C, the cocrystal structure of GlpG with DFP. The inhibitor is represented by a space-filling model. The L5 cap is disordered in the cocrystal structure.
