FIGURE 3.
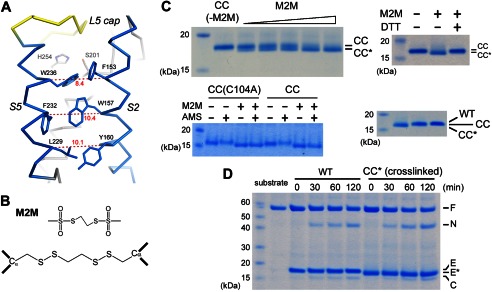
Cross-linking TM helices S2 and S5. A, distances (Å) between the Cα atoms are indicated for the 153/236, 157/232 and 160/229 residue pairs at the interface between S2 and S5. In this view, the Ser-His catalytic dyad is behind Phe-153 and Trp-236. B, the chemical structures of M2M and its reaction product with a pair of cysteines. C, cross-linked GlpG migrated faster on SDS-polyacrylamide gel. The M2M concentrations were 5, 10, 20, 30, and 70 μm. CC, the F153C/W236C double mutant; CC*, the cross-linked double mutant; CC(C104A), the C104A/F153C/W236C triple mutant; WT, wild-type. In the lower right panel, the wild-type protease also migrated slower than the cross-linked mutant. D, the cross-linked double mutant efficiently cleaved the maltose-binding protein-Gurken-thioredoxin fusion protein substrate in detergent solution. F, full-length substrate; N, N-terminal cleavage fragment; C, C-terminal fragment; E, enzyme; E*, cross-linked F153C/W236C.
