FIGURE 6.
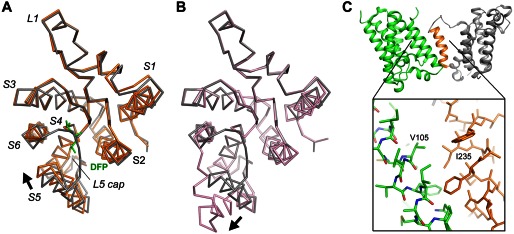
Comparison of the movement of S5. A, the GlpG-DFP cocrystal structure (orange) is superimposed onto the apo structure (gray) based on the Cα atoms of the central S4 helix (residues 201–216). The inhibitor is shown as a stick model (green). The arrow indicates the direction of the movement of S5. This image corresponds to a view from the extracellular side onto the membrane plane. B, a similar comparison between the open conformation (pink) and the closed conformation (gray). C, the heavily tilted S5 helix (orange) of one GlpG molecule (gray) interacts extensively with a neighboring molecule (green) in the crystal lattice. Favorable hydrophobic interactions, e.g. between Ile-235 and Val-105, may have caused the top of S5 to separate from the rest of the protein.
