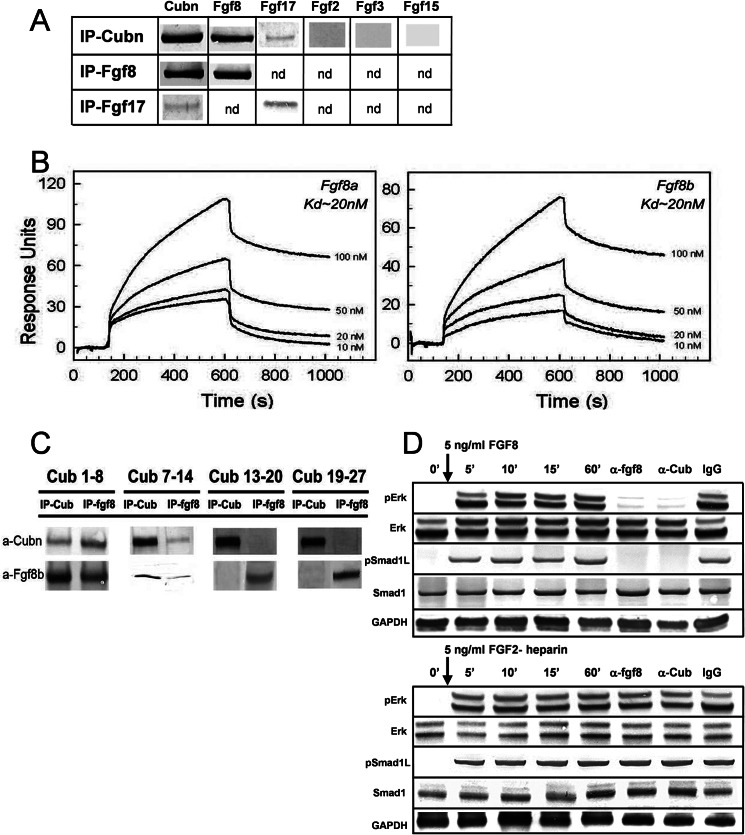
FIGURE 5.
Cubn binds Fgf8 with high affinity and contributes to Fgf8 signaling in vitro. A, co-immunoprecipitation (IP) of Fgf8 and the structurally similar Fgf17 with Cubn; Fgf2, Fgf3, or Fgf15 do not interact with Cubn. B, SPR analysis on immobilized Cubn. Fgf8a and Fgf8b bind with equally high affinity to Cubn. C, immunoprecipitation of the “mini-proteins” CUB1–8, -7–14, -13–20, and -19–27 preincubated with recombinant Fgf8b without heparin and with anti-Fgf8b and anti-Cubn antibodies. Fgf8b preferentially co-immunoprecipitates with CUB1–8 and CUB7–14. D, incubation of serum-deprived BN/MSV cells with either 5 ng/ml of recombinant Fgf8b without heparin or recombinant Fgf2-heparin results in similar phosphorylation of ERK1/2 and Smad1Linker. Anti-Cubn antibodies or anti-Fgf8b antibodies block the phosphorylation of ERK1/2 and Smad1Linker specifically in Fgf8b-treated cells. Control IgG has no effect. Endogenous Smad1 and ERK1/2 levels are shown for comparison, and GAPDH is used as loading control.