FIGURE 3.
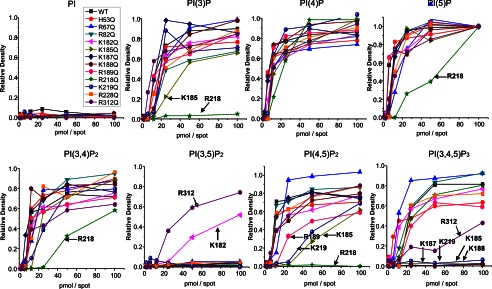
The interaction of individual mutant Kir 2.1 to the PIP Arrays. Mutant Kir2.1 protein bound to the PIP Arrays was probed with an anti-His antibody. The raw images are shown in supplemental Fig. S2. Densitometry measurements from PIP Arrays were internally normalized to density measured for the 100 pmol spot of PI(5)P and are plotted for lipids individually. The mutations that caused >50% reduction in binding compared with WT Kir2.1 are designated by an arrow and residue name. These data indicate that for each PIP, it is a different subset of residues that when mutated to Gln (Q) disrupts channel binding. This suggests that these ligands orient differently in the binding pocket, thereby interacting with different subset of residues, which may explain why they do not equivalently trigger activation in Kir2.1 channels.
