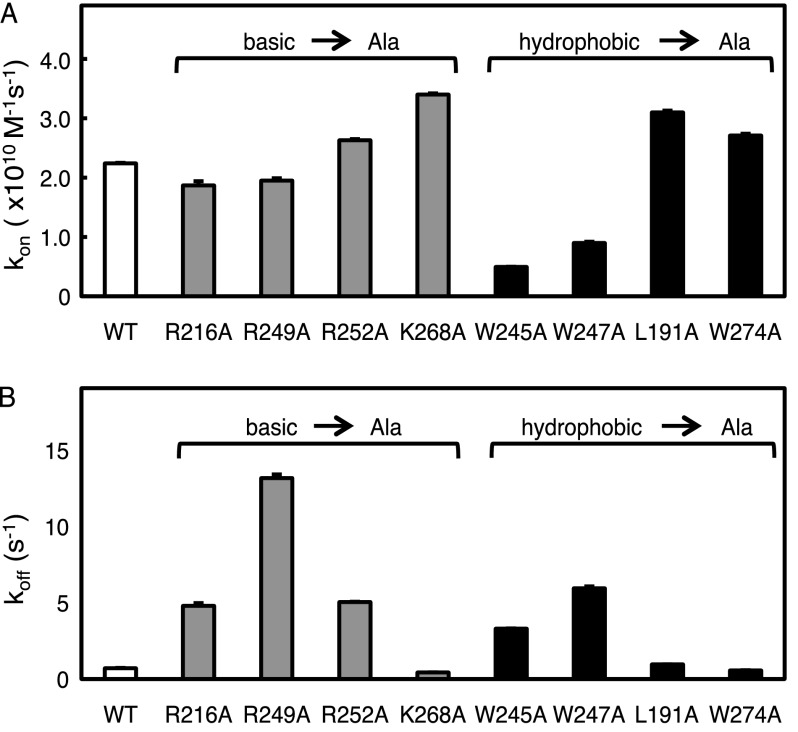
FIGURE 2.
Basic- or hydrophobic-to-neutral mutations on the C2 domain affect its recruitment to and retention on lipid vesicles. A, the binding of purified C2 domain (wild-type, R216A, R249A, R252A, K268A, W245A, W247A, L191A, or W274A) to anionic phospholipid vesicles was measured by FRET association stopped-flow fluorescence spectroscopy. The C2 domain protein (0.2–0.5 μm) was rapidly mixed with equal volumes of increasing concentrations of dansyl-labeled anionic phospholipid vesicles (PS/PC/dPE, 35:60:5 mol ratio) in the presence of 200 μm Ca2+ and 150 mm NaCl, as described under “Experimental Procedures.” The dansyl emission was measured over time, and the kobs was determined using a monophasic fit. The kon was calculated from the slope of the linear plot of kobs versus vesicle concentration. Data represent the weighted average ± S.E. (error bars) of 3–7 independent experiments. B, the binding of purified C2 domain (wild type, R216A, R249A, R252A, K268A, W245A, W247A, L191A, or W274A) to anionic phospholipid vesicles was measured by FRET dissociation stopped-flow analysis. The C2 domain protein (0.2–0.5 μm) was incubated for at least 15 min with dansyl-labeled phospholipid vesicles (PS/PC/dPE, 35:60:5 mol ratio). The protein and labeled lipid were then rapidly mixed with equal volumes of a 10-fold higher concentration of unlabeled anionic phospholipid vesicles (PS/PC, 40:60 mol ratio) in the presence of 200 μm Ca2+ and 150 mm NaCl. The dansyl emission was measured as a function of time, and the koff was determined using a monophasic fit. Data represent the weighted average ± S.E. of 3–7 independent experiments.