Fig. 2.
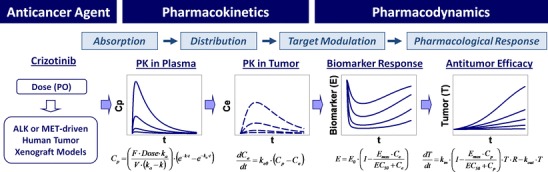
PKPD modeling summary of crizotinib-mediated target modulation and antitumor efficacy in human tumor xenograft models. C p, plasma concentration; F, oral bioavailability; k a, absorption rate constant; V, volume of distribution; k, elimination rate constant; t, time after dosing; C e, effect-site concentration; k e0, rate constant for equilibration with the effect site; E, biomarker response ratio to baseline (E 0); EC 50, concentration causing 50% of maximum effect (E max); T, tumor volume; R, logistic function (1-T/T ss), where T ss is a maximum sustainable tumor volume (R = 1 for exponential growth model)
