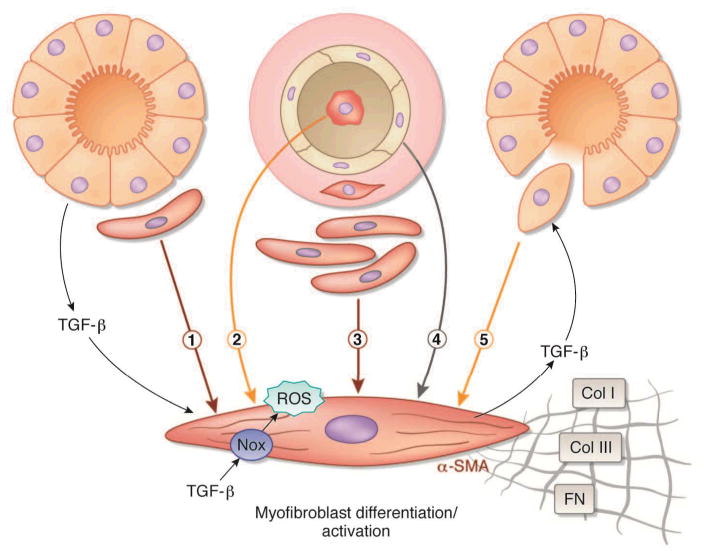
Figure 1. Proposed origin(s) of interstitial myofibroblasts during fibrosis.
Proposed origin(s) of interstitial myofibroblasts during fibrosis. Myofibroblasts responsible for interstitial expansion and structural damage during progressive organ injury have been proposed to be derived from one or more sources: (1) activation of resident fibroblasts or pericytes, (2) infiltration of circulating bone marrow-derived fibrocytes, (3) expansion of perivascular adventitial fibroblasts, (4) endothelial–mesenchymal (EndoMT) transition, and/or (5) epithelial-to-mesenchymal transition (EMT). Transforming growth factor-beta (TGF-β) released via paracrine or autocrine pathways induces myofibroblast differentiation expressed by the acquisition of an alpha-smooth muscle actin (α-SMA) phenotype and consequent synthesis of mesenchymal matrix proteins collagen type I (Col I), collagen type III (Col III), and fibronectin EIIIA (FN). Nox NAD(P)H oxidase (Nox4)-derived reactive oxygen species (ROS) have a central role in TGF-β signaling of myofibroblast differentiation.