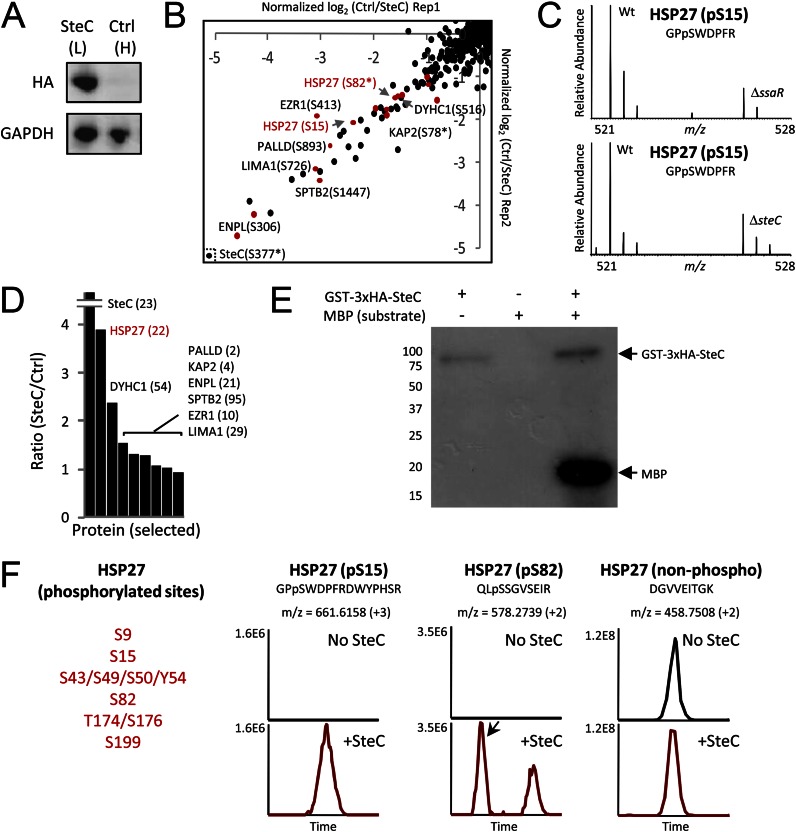
Fig. 4.
Quantitative screens to identify molecular targets of SteC. A, HeLa cells (L, light form) were transfected with 2×HA-SteC, whereas no transfection was performed for control HeLa cells (H, heavy). Expressions of SteC and GAPDH (control) were confirmed by immunoblotting. B, phosphoproteomics screen for discovery of substrates. The x and y axis show relative phosphorylation changes (log2-transformed H/L) of peptides upon SteC transfection in replicate 1 and in replicate 2, respectively. Red dots represent phosphopeptides from actin-associated proteins. C, phosphorylation change of HSP27 Ser-15 in infection experiments (8 h post-infection). WT Salmonella but not ΔssaR Salmonella (top panel) and ΔsteC Salmonella (bottom panel) induces HSP27 Ser-15 phosphorylation in HeLa. D, in the IP-MS screen for identifying binding partners of SteC, SteC-specific binding partners are identified by having an SteC/ctrl ratio significantly greater than 1. Only selected proteins (SteC and actin-associated proteins found in B) are shown, with the number of identified unique peptides in parentheses. E and F, in vitro kinase assay. E, GST-3×HA-SteC was incubated with a general kinase substrate myelin basic protein (MBP) and [γ-32P]ATP. Proteins are separated by SDS-PAGE and detected by autoradiography. F, after an in vitro reaction of recombinant human HSP27 with or without GST-3×HA-SteC, proteins are subjected to tryptic digestion, followed by LC-MS/MS analysis. At least six sites in HSP27 were phosphorylated by the SteC based on extracted ion chromatograms of the identified peptides.