Figure 2.
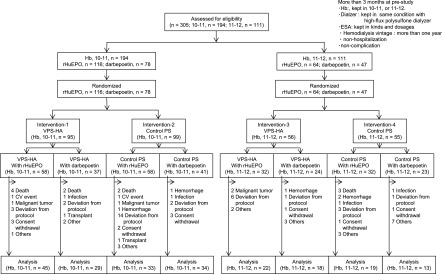
Patient disposition. Each patient was allocated to target hemoglobin range depending on one’s prestudy level of hemoglobin measured for 3 months, and the target hemoglobin level was kept during this trial. Death, cardiovascular, cerebrovascular, malignant tumor, and others as content; deviation from protocol, changing the type of erythropoiesis-stimulating agent (ESA), inadequate ESA dose, and dialyzer related as content; Hb, hemoglobin; hemorrhage, gastrointestinal hemorrhage; PS-con, type 4 polysulfone membrane; rHuEPO, human recombinant erythropoietin; transplant, renal transplantation, others, or change of hospital (patient factor); VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane; 10.0–10.9, hemoglobin range of 10.0–10.9 g/dl as target hemoglobin; 11.0–11.9, hemoglobin range of 11.0–11.9 g/dl as target hemoglobin.
