Summary
Background and objective
The risk of graft loss after pediatric kidney transplantation increases during late adolescence and early adulthood, but the extent to which this phenomenon affects all recipients is unknown. This study explored interactions between recipient factors and this high-risk age window, searching for a recipient phenotype that may be less susceptible during this detrimental age interval.
Design, setting, participants, & measurements
With use of Scientific Registry of Transplant Recipients data from 1987 to 2010, risk of graft loss across recipient age was quantified using a multivariable piecewise-constant hazard rate model with time-varying coefficients for recipient risk factors.
Results
Among 16,266 recipients, graft loss during ages ≥17 and <24 years was greater than that for both 3–17 years (adjusted hazard ratio [aHR], 1.61; P<0.001) and ≥24 years (aHR, 1.28; P<0.001). This finding was consistent across age at transplantation, sex, race, cause of renal disease, insurance type, pretransplant dialysis history, previous transplant, peak panel-reactive antibody (PRA), and type of induction immunosuppression. The high-risk window was seen in both living-donor and deceased-donor transplant recipients, at all levels of HLA mismatch, regardless of centers’ pediatric transplant volume, and consistently over time. The relationship between graft loss risk and donor type, PRA, transplant history, insurance type, and cause of renal disease was diminished upon entry into the high-risk window.
Conclusions
No recipient subgroups are exempt from the dramatic increase in graft loss during late adolescence and early adulthood, a high-risk window that modifies the relationship between typical recipient risk factors and graft loss.
Introduction
Kidney transplants performed during adolescence have excellent 1-year graft survival but paradoxically lower long-term graft survival compared with transplant recipients of other ages (1). Whereas previous studies analyzed static age at transplantation as the marker for this age effect (2–5), a recent study by Foster et al. instead examined graft failure rates across current recipient age and found an increased risk of graft failure among 17- to 24-year-olds regardless of the age at which they received the transplant (6).
The ability of pediatric recipients to successfully traverse these high-risk ages therefore appears to be a more important determinant of graft survival than the specific age at which transplantation was performed. In other words, the period of adolescence and early adulthood, along with any factors during that time that may lead to graft loss, is not specific only to transplantations performed at those ages but rather a high-risk “age window” through which all pediatric kidney transplant recipients must eventually pass. However, the extent to which this age window is equally detrimental to all recipients is unknown. For example, patients with the highest immunologic risk and those with the greatest barriers to successful transition between pediatric and adult posttransplant care could potentially experience an exaggerated increase in the risk of graft loss during these ages. In addition, this finding could vary dramatically across transplant centers or be biased by changes in graft survival over time.
The objective of this study was to develop a flexible statistical model that would allow us to (1) determine whether any phenotype of recipient is protected against the period of increased graft loss seen during late adolescence and early adulthood and (2) examine potential interactions between the high-risk age window and other risk factors for graft loss after pediatric kidney transplantation.
Materials and Methods
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR), a national registry of all solid organ transplants. The SRTR includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (7). The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Study Population
All pediatric (recipient <18 years of age) kidney-only transplant recipients between January 1987 and July 2010 were identified in the SRTR. Cause of renal disease was categorized as FSGS, other glomerular diseases, congenital anomalies of the kidney and urinary tract, or other/missing diagnosis on the basis of clinical knowledge and precedent set by the SRTR program-specific regression models (available at www.srtr.org). Induction immunosuppression was categorized as antilymphocyte therapy (antithymocyte globulin [Thymoglobulin], muromonab-CD3 [OKT3], or alemtuzumab [Campath]), IL-2 inhibitors, or no induction.
Outcome Ascertainment
Death-censored graft survival was defined as the time between transplantation and graft loss (marked by retransplantation or a return to dialysis) or last date of follow-up with a functioning graft, with censoring for death and administrative end of study. Death-censored graft failure rates were analyzed within various time intervals; specifically, 7-year graft failure rates were calculated to determine the likelihood of graft survival to age 24 given a functioning graft at age 17. Death ascertainment was supplemented by linkage to the Social Security Death Master File; death and graft loss ascertainment were also supplemented by linkage to data from the Centers for Medicare & Medicaid Services.
Hazard Plots
The risk of graft loss across age was graphically explored by plotting hazard functions against current recipient age (rather than the conventional posttransplant follow-up time). Age 0 (rather than date of transplantation) served as the time origin, with late entries into the risk set at each age of transplantation. The hazard function then provided the current graft failure rate at a given age conditional on graft survival up to that age.
Piecewise-constant Hazard Rate Model
A piecewise-constant hazard rate model was used to quantify the hazard of graft loss across posttransplant age. This model is an exponential hazard model that assumes a constant hazard within predefined time segments and then estimates variation in hazard between those time segments (8). Given that the time axis in the analysis was current recipient age, the time segments therefore consisted of periods of age (rather than follow-up time), thus enabling a closer examination of the high-risk age window of ages 17–24 years in comparison to the ages before and after this window. Sensitivity analyses were performed with different comparison age segments, and inferences were unchanged.
A multivariable parameterization of the piecewise-constant hazard rate model allowed us to compare hazard dynamically between posttransplant age categories while adjusting for potential recipient (sex, race, insurance, diagnosis, dialysis history, transplant history, and peak panel-reactive antibody [PRA]), donor (living versus deceased, age, and race), transplant (HLA mismatch and year), and center-level (pediatric transplant volume) confounders.
Subgroup Analyses
By use of the piecewise-constant hazard rate model, the dynamic risk of graft loss across posttransplant age was quantified by strata of recipient (age at transplantation, sex, race, diagnosis, insurance, dialysis history, transplant history, peak PRA, and type of induction immunosuppression), transplant (donor type, HLA mismatch, and year of transplantation), and center (pediatric volume) characteristics. Centers’ pediatric volume was categorized into tertiles according to the number of transplantations performed during the study period in patients <18 years of age.
Effect Modifier Analysis
Time-varying coefficients were added to the piecewise-constant hazard rate model to allow the relationship between risk factors and the hazard of graft loss to vary across various age segments (i.e., the hazard ratio for a given variable could differ across age each category). Specifically, factors associated with a higher risk of graft loss (recipient race, cause of renal disease, insurance status, previous transplant, PRA, donor type, and HLA mismatch) were examined as time-varying coefficients. The addition of these time-varying coefficients enabled the assessment of which characteristics made recipients especially susceptible (or resilient) to the graft loss observed during a particular age period. Through this approach, it was possible to determine, for example, whether repeat transplant recipients, although probably at higher risk of graft loss across all ages, were disproportionately at even higher risk of graft loss during late adolescence and early adulthood.
Statistical Analyses
All tests were two sided, with statistical significance set at α = 0.05. Analyses were performed using Stata software, version 12.0/SE (Stata Corp., College Station, TX).
Results
Graft Loss by Recipient Age
During the study period, 16,266 pediatric kidney transplants were performed (Table 1). Using hazard plots to examine graft loss across current recipient age, the risk of graft loss was most pronounced during late adolescence and early adulthood, as expected. In patients with a functioning graft at age 17 years, 42.4% were in fact expected to lose the graft by age 24. After adjustment for recipient, donor, transplant, and center-level characteristics with a multivariable piecewise-constant hazard rate model, the hazard of graft loss during ages ≥17 and <24 years remained significantly greater than that during ages 3–17 years (adjusted hazard ratio [aHR], 1.61; 95% confidence interval [CI], 1.52–1.70; P<0.001) and ages ≥24 years (aHR, 1.28; 95% CI, 1.18–1.38; P<0.001).
Table 1.
Recipient, donor, and transplant characteristics for pediatric (age <18 years) kidney transplantations performed between 1987 and 2010 (n=16,266)
| Variable | Data |
|---|---|
| Recipient | |
| Mean age ± SD (yr) | 11.1 ± 5.1 |
| Female | 40.9 |
| Race | |
| White | 58.3 |
| African American | 18.6 |
| Other | 23.1 |
| Cause of renal disease | |
| FSGS | 10.4 |
| Other glomerular | 14.6 |
| CAKUT | 34.5 |
| Other/missing | 40.5 |
| Public insurance | 64.6 |
| Preemptive status | 17.5 |
| Previous transplant | 11.8 |
| Peak PRA | |
| 0%–20% | 85.6 |
| 21%–80% | 10.3 |
| 81%–100% | 4.0 |
| Induction immunosuppression | |
| Antilymphocyte | 30.6 |
| IL-2 inhibitor | 25.5 |
| None | 43.9 |
| Donor | |
| Mean age ± SD (yr) | 30.0 ± 12.8 |
| Race | |
| White | 69.0 |
| African American | 12.6 |
| Other | 18.4 |
| Donor type (% living) | 49.3 |
| Transplant | |
| HLA mismatch | |
| 0 | 5.0 |
| 1–3 | 53.2 |
| 4–6 | 41.8 |
Unless otherwise noted, values are percentages. CAKUT, congenital anomalies of the kidney and urinary tract; PRA, panel-reactive antibody.
Subgroup Analyses: By Recipient Characteristics
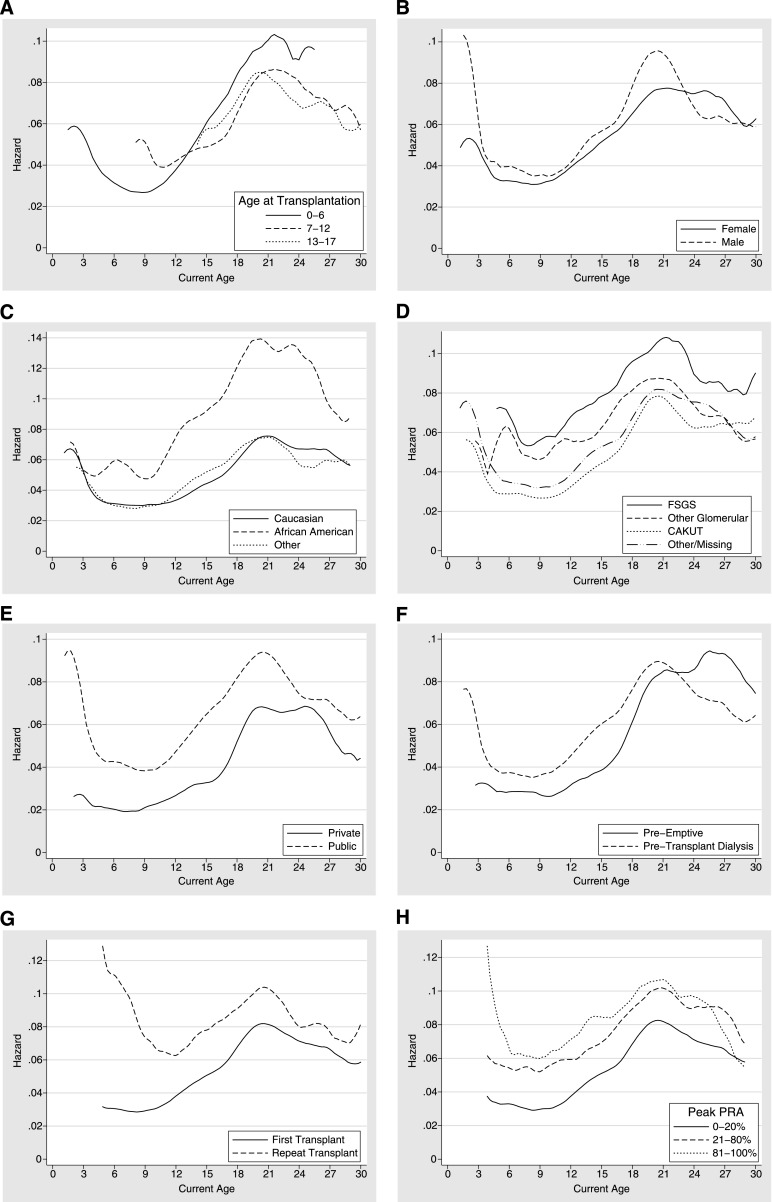
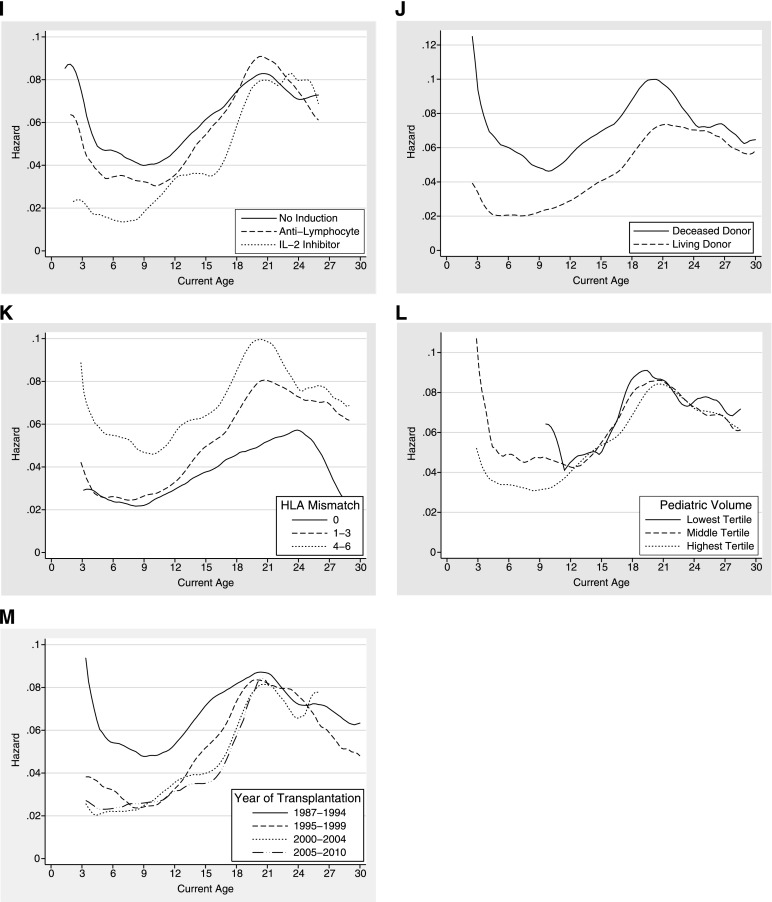
This increase in the hazard of graft loss during late adolescence and early adulthood was seen regardless of the age at transplantation (Figure 1A and Table 2). In addition, the increased risk of graft loss during ages 17–24 years was consistent across recipient sex, race, cause of renal disease, insurance type, pretransplant dialysis history, transplant history, peak PRA, and type of induction immunosuppression (Figure 1, B–I and Table 2).
Figure 1.
Hazard of graft loss across current recipient age among pediatric kidney transplant recipients. The hazard provides the current graft failure rate at a given age conditional on graft survival up to that age. Results are stratified by (A) age at transplantation, (B) sex, (C) race, (D) cause of renal disease, (E) insurance, (F) dialysis history, (G) transplant history, (H) peak panel-reactive antibody, (I) induction immunosuppression, (J) donor type, (K) HLA mismatch, (L) pediatric volume of transplant center, and (M) year of transplantation.
Table 2.
Adjusted hazard ratios comparing graft loss during ages 17–24 years with ages preceding this window (ages 3–17 years) and graft failure rates across the 17- to 24-year age window, stratified by subgroup
| Variable | Adjusted Hazard Ratio (95% Confidence Interval) | P Value | Grafts Lost, Age 17–24 yr (%)a |
|---|---|---|---|
| Age at transplant | |||
| 0–6 yr | 2.03 (1.72–2.40) | <0.001 | 49.8 |
| 7–12 yr | 1.57 (1.41–1.73) | <0.001 | 42.7 |
| 13–17 yr | 1.26 (1.15–1.37) | <0.001 | 41.7 |
| Sex | |||
| Female | 1.69 (1.56–1.84) | <0.001 | 45.0 |
| Male | 1.54 (1.44–1.66) | <0.001 | 40.6 |
| Race | |||
| White | 1.66 (1.54–1.79) | <0.001 | 39.0 |
| African American | 1.55 (1.39–1.72) | <0.001 | 59.6 |
| Other | 1.61 (1.42–1.81) | <0.001 | 39.9 |
| Cause of renal disease | |||
| FSGS | 1.32 (1.13–1.53) | <0.001 | 50.3 |
| Other glomerular | 1.36 (1.20–1.55) | <0.001 | 46.5 |
| CAKUT | 1.87 (1.70–2.07) | <0.001 | 38.6 |
| Other/missing | 1.62 (1.49–1.76) | <0.001 | 41.7 |
| Insurance | |||
| Public | 1.50 (1.41–1.59) | <0.001 | 45.5 |
| Private | 2.00 (1.79–2.24) | <0.001 | 36.0 |
| Dialysis history | |||
| Preemptive | 2.13 (1.85–2.45) | <0.001 | 44.6 |
| Pretransplant dialysis | 1.53 (1.45–1.63) | <0.001 | 44.0 |
| Transplant number | |||
| First transplant | 1.69 (1.59–1.79) | <0.001 | 41.5 |
| Repeat transplant | 1.25 (1.09–1.43) | <0.001 | 48.6 |
| Peak PRA | |||
| 0%–20% | 1.67 (1.57–1.77) | <0.001 | 41.6 |
| 21%–80% | 1.46 (1.25–1.71) | <0.001 | 50.1 |
| 81%–100% | 1.26 (0.99–1.61) | 0.07 | 50.9 |
| Induction | |||
| Antilymphocyte | 1.65 (1.49–1.82) | <0.001 | 44.3 |
| IL-2 inhibitor | 2.17 (1.88–2.50) | <0.001 | 39.3 |
| None | 1.44 (1.34–1.55) | <0.001 | 42.2 |
| Donor type | |||
| Living | 1.98 (1.82–2.15) | <0.001 | 38.8 |
| Deceased | 1.38 (1.29–1.48) | <0.001 | 46.7 |
| HLA mismatch | |||
| 0 | 1.48 (1.12–1.95) | 0.005 | 31.1 |
| 1–3 | 1.78 (1.65–1.92) | <0.001 | 41.2 |
| 4–6 | 1.44 (1.32–1.56) | <0.001 | 46.9 |
| Pediatric volume of transplant center | |||
| Lowest tertile | 1.39 (0.82–2.37) | 0.2 | 48.7 |
| Middle tertile | 1.50 (1.30–1.73) | <0.001 | 44.3 |
| Highest tertile | 1.63 (1.54–1.73) | <0.001 | 42.0 |
| Year of transplantation | |||
| 1987–1994 | 1.31 (1.21–1.42) | <0.001 | 43.4 |
| 1995–1999 | 1.88 (1.69–2.09) | <0.001 | 43.2 |
| 2000–2004 | 1.97 (1.73–2.23) | <0.001 | 38.8 |
| 2005–2010 | 1.78 (1.47–2.17) | <0.001 | – |
Each row represents a separate subgroup analysis. CAKUT, congenital anomalies of the kidney and urinary tract; PRA, panel-reactive antibody.
Death-censored graft failure rate across the 17- to 24-year age window among grafts still functioning at age 17.
Subgroup Analyses: By Donor, Transplant, and Center Characteristics
The increased risk of graft loss during late adolescence and early adulthood was seen in both living-donor and deceased-donor transplant recipients and at all levels of HLA mismatch (Figure 1, J and K, and Table 2). This finding was not diminished among centers with the highest pediatric volumes (Figure 1L and Table 2) and has not improved over time (Figure 1M and Table 2).
Effect Modifier Analysis
Some recipient risk factors had a similar relationship with the hazard of graft loss before, during, and after the high-risk window of 17–24 years (Table 3). For example, the relative increase in graft loss for African American recipients (versus white recipients) during the 17- to 24-year age window (aHR, 1.58; 95% CI, 1.44–1.74; P<0.001) was not significantly different than the age interval before (aHR, 1.71; 95% CI, 1.55–1.89; P<0.001) or after (aHR, 1.59; 95% CI, 1.31–1.92; P<0.001) that age window. In addition, the relationship between HLA mismatch and the hazard of graft loss was similar before, during, and after the high-risk age window (Table 3).
Table 3.
Adjusted hazard ratios for risk factors before, during, and after the high-risk age window (ages 17–24 years)
| Variable | Adjusted Hazard Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| Age 3–17 yr | Age 17–24 yr | Age >24 yr | |
| Race | |||
| African American (vs. white) | 1.71 (1.55–1.89) | 1.58 (1.44–1.74) | 1.59 (1.31–1.92) |
| Cause of renal disease (vs. other/missing) | |||
| FSGS | 1.56 (1.38–1.77) | 1.22 (1.08–1.38)a | 1.18 (0.89–1.55) |
| Other glomerular | 1.25 (1.11–1.41) | 1.08 (0.98–1.19)b | 0.89 (0.75–1.06) |
| CAKUT | 0.85 (0.77–0.93) | 1.02 (0.94–1.12) | 1.07 (0.90–1.28) |
| Insurance | |||
| Public (vs. private) | 1.44 (1.30–1.59) | 1.06 (0.97–1.16)c | 0.82 (0.69–0.99) |
| Transplant number | |||
| Repeat transplant (vs. first transplant) | 1.53 (1.37–1.71) | 1.15 (1.03–1.28)c | 1.06 (0.87–1.30) |
| Peak PRA (vs. 0%–20%) | |||
| 21%–80% | 1.21 (1.07–1.36) | 1.04 (0.92–1.16) | 1.12 (0.91–1.37) |
| 81%–100% | 1.36 (1.13–1.64) | 1.04 (0.87–1.25)d | 0.86 (0.59–1.25) |
| Donor type | |||
| Living (vs. deceased) | 0.57 (0.52–0.63) | 0.81 (0.75–0.89)c | 1.13 (0.97–1.30) |
| HLA mismatch (vs. 0) | |||
| 1–3 | 1.14 (0.96–1.37) | 1.33 (1.15–1.55) | 1.57 (1.22–2.03) |
| 4–6 | 1.46 (1.22–1.76) | 1.37 (1.18–1.61) | 1.31 (0.99–1.72) |
PRA, panel-reactive antibody.
P=0.004; statistically significant effect modification (comparing age 17–24 to age 3–17).
P=0.05; statistically significant effect modification (comparing age 17–24 to age 3–17).
P<0.001; statistically significant effect modification (comparing age 17–24 to age 3–17.
P=0.03; statistically significant effect modification (comparing age 17–24 to age 3–17).
In contrast, the relationship between several recipient variables and the risk of graft loss was diminished during the high-risk window of 17–24 years (Table 3). The increased hazard associated with a prior kidney transplant (versus patients receiving first transplants) was more dramatic during ages 3–17 years (aHR, 1.53; 95% CI, 1.37–1.71; P<0.001) than during ages 17–24 years (aHR, 1.15; 95% CI, 1.03–1.28; P=0.01). Likewise, the increased hazard associated with public insurance (versus patients with private insurance) was lost when advancing from ages 3–17 years (aHR, 1.44; 95% CI, 1.30–1.59; P<0.001) to ages 17–24 years (aHR, 1.06; 95% CI, 0.97–1.16; P=0.2). Finally, the increased risk of having FSGS or another glomerular disease, as well as having an elevated peak PRA, was also diminished when moving from ages 3–17 to ages 17–24 years (Table 3).
The graft survival advantage for a living-donor transplant compared with a deceased-donor transplant was also attenuated by the high-risk window of 17–24 years (Table 3). The relative decrease in the risk of graft loss for living-donor transplants (versus deceased-donor transplants) diminished when moving from ages 3–17 (aHR, 0.57; 95% CI, 0.52–0.63; P<0.001) to ages 17–24 years (aHR, 0.81; 95% CI, 0.75–0.89; P<0.001) and no longer remained after age 24 (aHR, 1.13; 95% CI, 0.97–1.30; P=0.1).
Discussion
This national study of more than 16,000 pediatric kidney transplants closely investigated the changing risk of graft loss across recipient age using a flexible statistical model to determine whether susceptibility to the high-risk age window varies and to evaluate how recipient risk factors interact with this age window. The increased risk of graft loss during late adolescence and early adulthood unfortunately appears to be a universal phenomenon, generally consistent across all the recipient, donor, transplant, and center characteristics that were examined. No recipient groups appeared to be unharmed by this high-risk window; in fact, recipients with certain traditionally favorable characteristics (those with diagnoses that are less likely to recur, with private insurance, with low PRA, receiving a first transplant, and receiving a living-donor transplant) seemed to be most vulnerable during this detrimental age interval (relatively speaking, compared with those patients during other age intervals). Essentially the high-risk age window lessened the favorable effect of these variables on graft survival (or alternatively, lessened the unfavorable impact of their alternatives).
Our findings are consistent with and build on the findings of Foster et al., who reported an increased graft failure rate among 17- to 24-year-olds regardless of the age at transplantation (6). Previous studies have reported decreased graft survival among patients receiving transplants in their adolescent years (i.e., age at transplantation) (1–5,9,10). However, graft failure risk appears to be more closely related to current posttransplant age at follow-up rather than age at transplantation, and adolescent age at transplantation may be important only to the degree that it marks imminent entry into the late teens and early twenties (i.e., posttransplant age at follow-up). Of important note, the patients in this study who underwent transplantation between ages 13 and 17 years did not have a more exaggerated risk of graft loss during the high-risk window than patients who had transplantation at younger ages. Patients undergoing transplantation at all ages had a dramatically increased risk of graft loss during the high-risk age window, but adolescent recipients in particular did not experience a more dramatic risk of graft loss during late adolescence and early adulthood.
The increased risk of graft loss during late adolescence and early adulthood may be due to adolescents’ lack of adherence to immunosuppression (11–17). Given the extension of the high-risk age window into early adulthood, the effect of this nonadherence on graft survival is probably delayed, or, alternatively, the lack of adherence may also extend into early adulthood. Of note, we found that the use of induction immunosuppression did not appear to lessen the high risk of graft loss during late adolescence and early adulthood. In addition, the high-risk age window was consistent across varying levels of immunologic risk as measured by peak PRA and HLA mismatch.
Nonadherence to immunosuppression during late adolescence and early adulthood may be exacerbated by concurrent alterations in health insurance coverage, which have also been linked to poor outcomes after pediatric kidney transplantation (18–20). The SRTR unfortunately lacks the granularity with respect to insurance status and its changes over time that would allow an in-depth analysis of this factor. However, this study was able to show that recipients with both private and public insurance at the time of transplantation did experience an increased risk of graft loss during late adolescence and early adulthood.
Finally, transitions from pediatric to adult care may also contribute to this high-risk age window (15,21–25). Special clinics aimed at integrating pediatric and adult care to smooth these transitions appear to reduce graft failure rates (26). The SRTR does not adequately capture this transition process, but assuming that most transfers of care occur between ages 17 and 21 years, one would hypothesize an increased risk of graft loss beginning near those particular ages. If transfer of care were primarily responsible for the findings, one might also expect an even more dramatic increase in risk among patients who underwent transplantation at low-volume centers, where the transfer of care may be less established compared with high-volume centers that have greater experience at streamlining these transitions. However, this study did not support this effect.
This study’s finding that the high-risk window is consistent across all the subgroups examined may at first appear to be a disappointment. The identification of a select few recipient subgroups at high risk of graft loss during these ages not only would have enabled a focusing of provider efforts on the most vulnerable patients but could also have shed light on the specific causes that may be creating this high-risk age window. Indeed, it is certainly disappointing that we could not identify a single recipient phenotype that appears to gracefully pass through this high-risk age window. However, in terms of etiology, the universality of this finding across all recipient subgroups may suggest that an “all-of-the-above” category may be the true cause. In addition, some aspect of this phenomenon may be biologic, with the period of increased growth during adolescence leading to hyperfiltration injury, and thereby subsequent increased rates of graft loss, similar to the hyperfiltration injury thought to occur when kidneys from small donors are transplanted into large recipients (27).
This study is limited by the variables available for analysis with the SRTR. For example, the specific characteristics of the transition process between pediatric and adult posttransplant care is not well captured in the SRTR. Our study therefore could not examine in-depth the role of care transitions in the high-risk age window; it remains possible that a subgroup of recipients experiencing the most ideal transition of care could theoretically be less vulnerable during the high-risk age window. An additional limitation of this study with regard to use of the piecewise-constant hazard rate model is the somewhat arbitrary nature of the choice of age segments. The age window of 17–24 years was chosen empirically and confirmed by the work of Foster et al. (6), which identified those particular ages as having an increased risk of graft loss; however, the comparison age segments before and after this risk window could have been narrowed or widened. On the basis of sensitivity analyses, however, such choice of different age segments, although changing the exact point estimates for the hazard comparisons, did not change the inferences from our study.
In conclusion, we compared the substantial increase in the risk of graft loss during late adolescence and early adulthood across various patient- and center-level characteristics. No particular recipients appear to be exempt from this high-risk age window, so much so that the favorable effect of some factors (such as having a living donor) on graft survival appears to be dampened or even completely eliminated because of this particular age interval. Because all pediatric recipients must eventually traverse this late adolescent and early adult age window, our study underscores this high-risk window as a prime area for intervention to maximize continuity of care, insurance coverage, and immunosuppression adherence in order to improve long-term pediatric graft survival.
Disclosures
None.
Acknowledgments
This research was presented in preliminary form as an oral abstract at the 2012 American Society of Transplant Surgeons State of the Art Winter Symposium and the 2012 American Transplant Congress. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Magee JC, Bucuvalas JC, Farmer DG, Harmon WE, Hulbert-Shearon TE, Mendeloff EN: Pediatric transplantation. Am J Transplant 4[Suppl 9]: 54–71, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Smith JM, Ho PL, McDonald RA, North American Pediatric Renal Transplant Cooperative Study : Renal transplant outcomes in adolescents: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 6: 493–499, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Gjertson DW, Cecka JM: Determinants of long-term survival of pediatric kidney grafts reported to the United Network for Organ Sharing kidney transplant registry. Pediatr Transplant 5: 5–15, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Hwang AH, Cho YW, Cicciarelli J, Mentser M, Iwaki Y, Hardy BE: Risk factors for short- and long-term survival of primary cadaveric renal allografts in pediatric recipients: A UNOS analysis. Transplantation 80: 466–470, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Harmon WE, McDonald RA, Reyes JD, Bridges ND, Sweet SC, Sommers CM, Guidinger MK: Pediatric transplantation, 1994-2003. Am J Transplant 5: 887–903, 2005 [DOI] [PubMed]
- 6.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA: Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92: 1237–1243, 2011 [DOI] [PubMed] [Google Scholar]
- 7.OPTN/SRTR 2010 Annual Data Report. Rockville, MD, Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR), 2011
- 8.Sorensen JB: STPIECE: Stata module to estimate piecewise-constant hazard rate models. Boston, MA, Statistical Software Components, Boston College Department of Economics, 1999 [Google Scholar]
- 9.Cecka JM, Gjertson DW, Terasaki PI, United Network for Organ Sharing : Pediatric renal transplantation: A review of the UNOS data. Pediatr Transplant 1: 55–64, 1997 [PubMed] [Google Scholar]
- 10.Kiberd JA, Acott P, Kiberd BA: Kidney transplant survival in pediatric and young adults. BMC Nephrol 12: 54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dew MA, Dabbs AD, Myaskovsky L, Shyu S, Shellmer DA, DiMartini AF, Steel J, Unruh M, Switzer GE, Shapiro R, Greenhouse JB: Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation 88: 736–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rianthavorn P, Ettenger RB: Medication non-adherence in the adolescent renal transplant recipient: A clinician’s viewpoint. Pediatr Transplant 9: 398–407, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Shellmer DA, Dabbs AD, Dew MA: Medical adherence in pediatric organ transplantation: What are the next steps? Curr Opin Organ Transplant 16: 509–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN: Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: A systematic review. Pediatr Transplant 14: 603–613, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Watson AR: Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol 14: 469–472, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Wolff G, Strecker K, Vester U, Latta K, Ehrich JH: Non-compliance following renal transplantation in children and adolescents. Pediatr Nephrol 12: 703–708, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S: Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant 9: 381–390, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Willoughby LM, Fukami S, Bunnapradist S, Gavard JA, Lentine KL, Hardinger KL, Burroughs TE, Takemoto SK, Schnitzler MA: Health insurance considerations for adolescent transplant recipients as they transition to adulthood. Pediatr Transplant 11: 127–131, 2007 [DOI] [PubMed] [Google Scholar]
- 19.White PH: Access to health care: Health insurance considerations for young adults with special health care needs/disabilities. Pediatrics 110: 1328–1335, 2002 [PubMed] [Google Scholar]
- 20.Schnitzler MA, Lentine KL, Burroughs TE, Irish WD, Brennan DC, Woodward RS: Consequences of the end of medicare coverage in pediatric renal transplant recipients [Abstract]. Am J Transplant 5: 563, 2005 [Google Scholar]
- 21.Chaturvedi S, Jones CL, Walker RG, Sawyer SM: The transition of kidney transplant recipients: A work in progress. Pediatr Nephrol 24: 1055–1060, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Magee JC, Thomas SE, Fredericks EM, Guidinger MK, Port FK, Kalbfleisch JD, Wolfe RA: Effect of recipient age and “transition” on graft loss in pediatric transplant recipients. Transplantation 82: 214, 2006 [Google Scholar]
- 23.Samuel SM, Nettel-Aguirre A, Hemmelgarn BR, Tonelli MA, Soo A, Clark C, Alexander RT, Foster BJ, Pediatric Renal Outcomes Canada Group : Graft failure and adaptation period to adult healthcare centers in pediatric renal transplant patients. Transplantation 91: 1380–1385, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Watson AR, Harden P, Ferris M, Kerr PG, Mahan J, Ramzy MF: Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA). Pediatr Nephrol 26: 1753–1757, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Foster BJ, Platt RW, Dahhou M, Zhang X, Bell LE, Hanley JA: The impact of age at transfer from pediatric to adult-oriented care on renal allograft survival. Pediatr Transplant 15: 750–759, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Harden PN, Walsh G, Bandler N, Bradley S, Lonsdale D, Taylor J, Marks SD: Bridging the gap: An integrated paediatric to adult clinical service for young adults with kidney failure. BMJ 344: e3718, 2012 [DOI] [PubMed] [Google Scholar]
- 27.el-Agroudy AE, Hassan NA, Bakr MA, Foda MA, Shokeir AA, Shehab el-Dein AB: Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am J Nephrol 23: 294–299, 2003 [DOI] [PubMed] [Google Scholar]