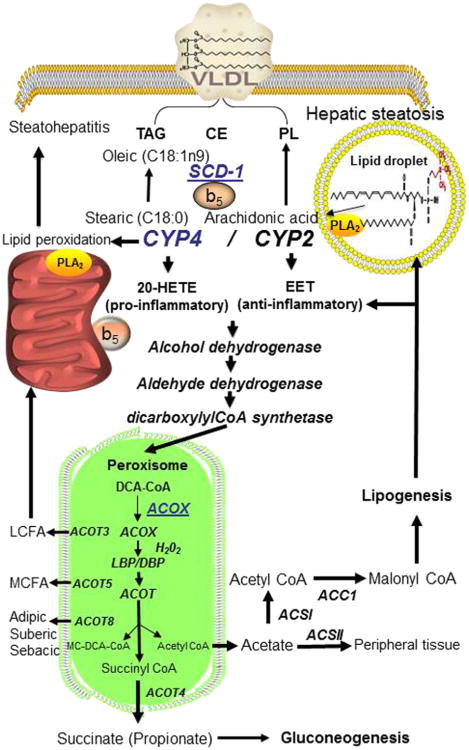
Figure 5.4. CYP4A and CYP2C P450s function in liver eicosanoid and fatty acid metabolism.
Fatty acids are delivered to hepatocytes for catabolism by the mitochondria or peroxisome β-oxidation systems or are used for the synthesis and export of triglycerides (TG), phospholipid (PL), cholesterol esters (CE), as very low density lipoprotein (VLDL) particles. Medium- and short-chain-length fatty acids are transported as acylcarnitine derivatives into mitochondria for complete β-oxidation to CO2. Long- and very-long-chain fatty acid CoA esters as well as eicosanoids are transported into the peroxisome where they undergo chain-shortening reactions and then transported as acylcarnitine derivatives to the mitochondria for complete oxidation or as free fatty acids by thioesterase 3, or 5 (ACOT3, 5). Under normal conditions, (5–10%) fatty acids are converted to dicarboxylic acids CoA (DCA-CoA) by CYP4A or CYP4F ω-hydroxylation. Starvation induces the ω-oxidation of fatty acids by 40% through PPARα induction of CYP4A genes. The dicarboxylic acids, adipyl-CoA (C6), sebacyl-CoA (C8) or suberyl-CoA (C10) are exported from peroxisome by the action of acyl-CoA thioesterase 8 (ACOT8), or when ACOT8 is inhibited by free CoASH, the FF-CoA is converted to succinyl-CoA, which is exported as succinate after removal of CoA by ACOT4. Succinate can function as an anaplerotic intermediate in the mitochondria for gluconeogenesis during starvation and excess acetate produced from acetyl-CoA can be used by peripheral tissues after conversion to acetyl-CoA by acetate-CoA synthetase (ACSII). In the fed state, long-chain fatty acids (LCFA) and very-long-chain fatty acids (VLCFA) are metabolized by peroxisome |3-oxidation to shorter chain products, which can be incorporated into phospholipids, cholesterol esters or triglycerides for export as VLDS and stored in peripheral tissues. Excessive acetate in the cytosol is converted to acetyl-CoA by hepatocyte acetate-CoA synthetase I (ACSI) and then converted to malonyl-CoA for fatty acid and cholesterol synthesis by ACC1. CYP4F gene induction by insulin is mediated by SREBP and saturated fatty acid-CoA activation of hepatocyte nuclear factor 4α (HNF4α). In the presence of excessive fatty acids, insulin will activate SREBP1c increasing the synthesis of stearoyl-CoA desaturase 1 (SCD-1) and converting palmitic (C16:0) and stearic (C18:0) acids to palmitoleate (C16:1) and oleic (C18:1), respectively, which are stored as triglycerides. Both CYP4 P450 and SCD-1 use cytochrome b5 and cytochrome b5 reductase in their catalytic cycle. The induction of CYP4A genes by high-fat-diet and an increase in SCD-1 with suppression of CYP4F genes may prevent the liver from lipotoxicity at the expense of steatosis and development of steatohepatitis. ACOX, acyl-CoA oxidase; LBP, L-bifunctional protein; DBP, D-bifunctional protein; TG, triglycerides; PL, phosphor\lipid; CE, cholesterol ester; WE, wax ester. (For color version of this figure, the reader is referred to the online version of this book).