Fig 7.
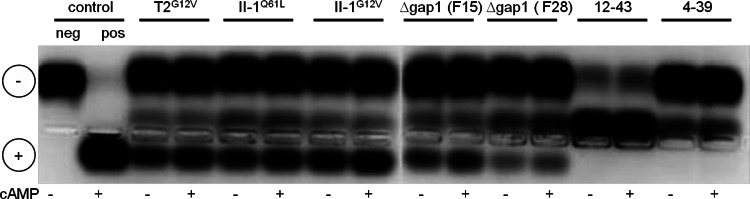
Examination of the cAMP-dependent PKA activity in protein extracts of wild-type (12-43, 4-39), constitutively active Ras (T2G12V, II-1G12V, II-1Q61L), and Δgap1 mutant (F15, F28) strains. PKA activity was assayed with a colored peptide (kemptide) that is phosphorylated by PKA. In the wild-type strains, no PKA activity was detectable even after the addition of cAMP. In contrast, the negative charge led to the migration of phosphorylated kemptide toward the positively charged electrode in all of the mutant strains, also without the addition of cAMP.
