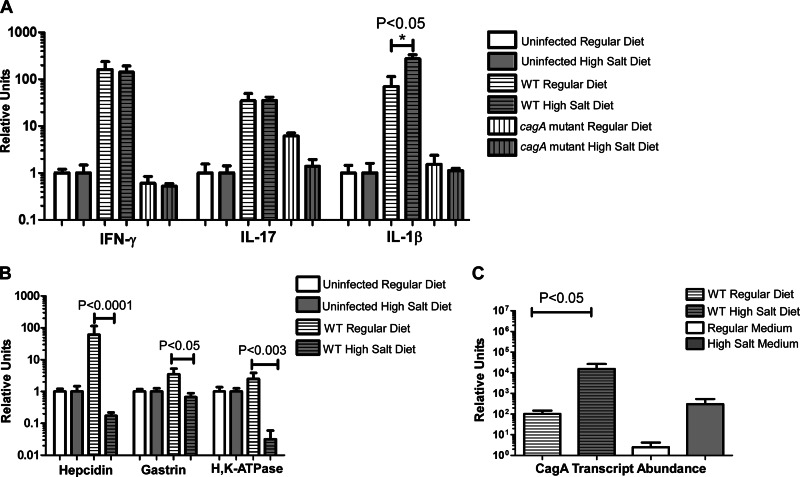
Fig 6.
Real-time RT-PCR analysis of the host immune response, modulators of gastric acid production, and H. pylori cagA in the gerbil stomach. For panels A and B, RNA extracts from the stomachs of 5 to 7 individual H. pylori-infected rodents were analyzed, along with RNA from uninfected control animals (same diet conditions) pooled into a single control. For panel C (analysis of cagA transcription), gastric RNA extracts were analyzed along with RNA derived from H. pylori grown in modified brucella broth alone (regular medium) or supplemented with 0.5% sodium chloride (high-salt medium). The results represent mean values based on analyses of 5 to 7 animals per group. (A) IL-1β, IL-17, and IFN-γ were induced by WT infection. IL-1β transcript abundance was significantly increased in WT-infected animals maintained on a high-salt diet compared to WT-infected animals maintained on a regular diet. (B) Hepcidin, gastrin, and H,K-ATPase are downregulated in the WT-infected high-salt-diet animals compared to the WT-infected regular-diet animals. For panels A and B, transcript abundance was normalized to GAPDH, and relative units were calculated as described in Materials and Methods. (C) cagA gene expression is elevated in WT-infected animals maintained on a high-salt diet compared to WT-infected animals maintained on a regular diet (P < 0.05). Similarly, cagA transcription was increased in bacteria grown in vitro in high-salt medium compared to bacteria grown in regular medium. For panel C, bacterial transcript levels were normalized to the corresponding 16S rRNA levels in each sample. Relative units were calculated as described in Materials and Methods.