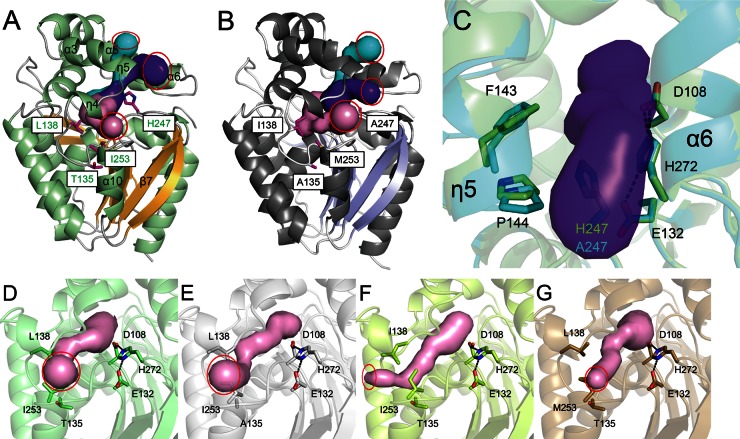
Fig 5.
Different amino acid residues lining the access tunnel between LinBMI and LinBUT. (A and B) The three access tunnels (pink, purple, and cyan) to the active site of wild-type LinBMI (A) or LinBUT (B). The catalytic triad residues (green) and amino acid residues (magenta) that are different between wild-type LinBMI and LinBUT are shown as stick models. The red circles represent the entrances of the access tunnels. (C) Superimposition between the wild type (light green) and the H247A mutant (cyan) of LinBMI. The tunnel (purple) observed in wild-type LinBMI is shown. The catalytic triad residues and the residues at positions 135, 138, and 253 are shown as sticks in the wild-type (D), T135A mutant (E), L138I mutant (F), or I253M mutant (G) structure of LinBMI. The red circles show the entrances of the access tunnels of the wild type and three mutants.