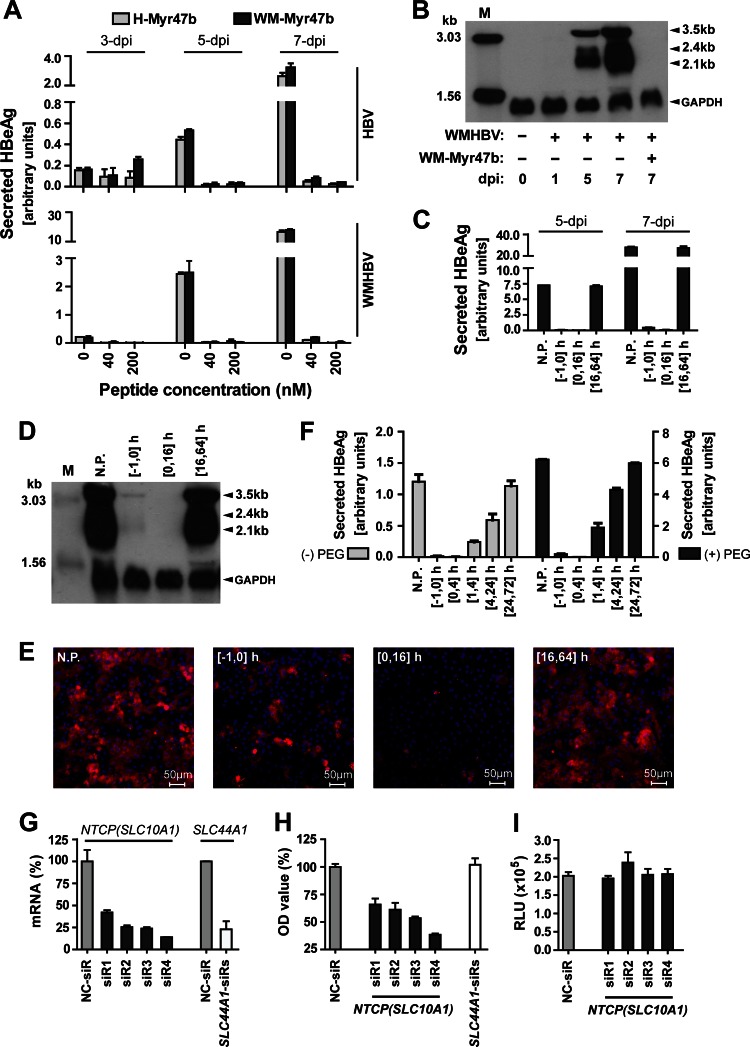
Fig 4.
Inhibition of WMHBV infection in PTHs by tsNTCP-binding peptide or tsNTCP-specific siRNAs. (A) Inhibition of HBV and WMHBV infection by peptide WM-Myr47b or H-Myr47b. PTHs were inoculated with Huh7-produced WMHBV at an MGE of 100 or HBV (genotype D) at an MGE of 200 in the presence of 4% PEG 8000 for 16 h. The indicated peptide was added immediately before inoculation and was present during the inoculation process. Cell culture supernatants were collected every 2 days, and secreted HBeAg was measured by ELISA. The relative quantity of HBeAg is presented as arbitrary units per 50 μl of culture supernatant, which was calculated by multiplying the optical density at 450 nm by the dilution factors. (B) PTHs in six-well plates were inoculated with WMHBV as in panel A. At 0, 1, 5, and 7 dpi, cells were homogenized for total RNA extraction. A 10-μg sample of total RNA was separated on a 1.5% agarose gel and then blotted to Hybond-N+ positively charged nylon membrane (GE Healthcare Life Sciences) by overnight capillary transfer in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Hybridization was performed at 52°C for 6 h with a random primed digoxigenin-dUTP (Roche Applied Science)-labeled probe covering the whole genome of WMHBV and a probe spanning human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript variant 1 mRNA nucleotides 181 to 690. Hybridized probes were detected with anti-digoxigenin antibody–alkaline phosphatase conjugate and CSPD chemiluminescence substrate (Roche Applied Science). The chemiluminescence signal was detected with BioMax Light chemiluminescence film (Sigma). Samples (100 pg) of denatured 3,032- and 1,554-bp PCR fragments of the WMHBV genome were included as markers. (C to E) Blocking of WMHBV infection by adding WM-Myr47b at different time points. PTHs were inoculated with WMHBV at an MGE of 100 in the presence of 4% PEG 8000 for 16 h. The WM-Myr47b peptide was added to PTHs at 200 nM and incubated for different times as indicated. The time of virus addition to cells is designated 0 h. N.P., no peptide treatment. (C) Cell culture supernatants were collected every 2 days, and secreted HBeAg was measured on the indicated days by ELISA. (D) At 7 dpi, infected PTHs in a six-well plate were homogenized for total RNA extraction and 10 μg of RNA was subjected to Northern blot analysis as in panel B. Lane M corresponds to 10 pg of denatured 3,032- and 1,554-bp PCR fragments from the WMHBV genome. (E) At 14 dpi, infected PTHs were fixed with 4% paraformaldehyde and intracellular HBcAg of WMHBV was stained with 5 μg/ml MAb 1C10 and then with Alexa 488-conjugated goat anti-mouse IgG (Life Technologies). Fluorescence images were captured with a Zeiss LSM 510 Meta confocal microscope. Red, WMHBV HBcAg; blue, cell nucleus. (F) WM-Myr47b-mediated inhibition of WMHBV infection at 4 h after inoculation. PTHs were inoculated with WMHBV at an MGE of 100 in the presence or absence of 4% PEG 8000 for 4 h. The WM-Myr47b peptide was added at 200 nM to PTHs and incubated for the times indicated. The time at which the virus was added to the cells was marked as 0 h. Secreted HBeAg was measured by ELISA at 5 dpi. N.P., no peptide treatment. (G) Validation of the knockdown efficiencies of siRNAs at the mRNA level. Freshly isolated PTHs were transfected with 20 nM siRNA targeting tsNTCP (SLC10A1: siR1, siR2, siR3, and siR4), or tsSLC44A1 (SLC44A1-siRs) or with a nontargeting control siRNA (NC-siR). Three days after transfection, PTHs were lysed for total RNA isolation and cDNA preparation. Tupaia NTCP (SLC10A1) and SLC44A1 mRNA levels were then determined by qRT-PCR with tsNTCP- and tsSLC44A1-specific primers, respectively. qRT-PCR was performed with the SYBR Premix Ex Taq kit (TaKaRa, Tokyo, Japan) on an ABI Fast 7500 RT-PCR system (Applied Biosystems). Data are presented as the relative mRNA levels of NTCP and SLC44A1 compared to that in cells transfected with the negative-control siRNA (NC-siR). (H) WMHBV infection of PTHs is reduced by tsNTCP knockdown. Freshly isolated PTHs were transfected as described for panel G. Three days after transfection, cells were inoculated with WMHBV at an MGE of 100 for 16 h. The culture medium was refreshed every 2 days, and secreted HBeAg was measured at 5 dpi. Data are presented as the relative optical density (OD) value compared to the optical density at 450 nm for HBeAg from cells transfected with the negative-control siRNA (NC-siR). (I) Infection with control virus lenti-VSV-G was not affected by tsNTCP knockdown. Freshly isolated PTHs were transfected as described for panel G. Three days after transfection, cells were inoculated with lenti-VSV-G for 16 h. The culture medium was refreshed every 2 days, and cells were lysed for luciferase activity detection at 5 dpi. RLU, relative luminescence units.