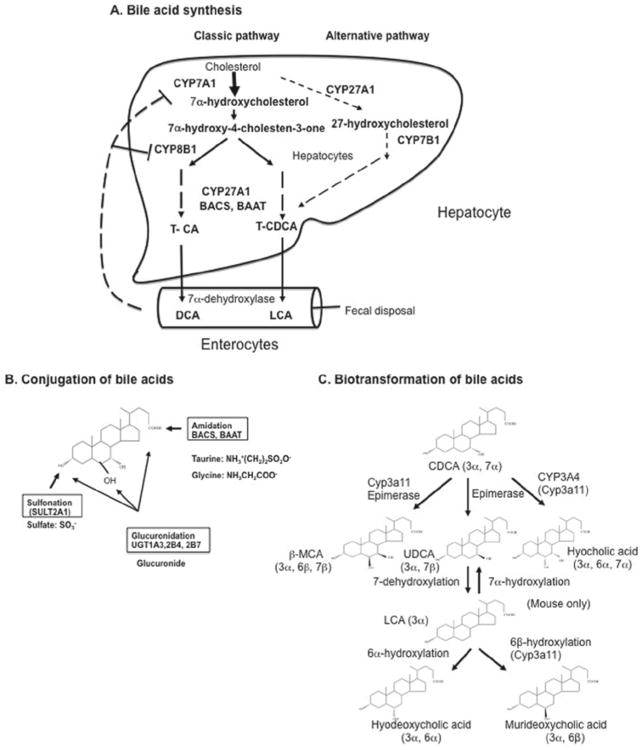
Figure 2.
Bile acid synthesis, conjugation, and biotransformation. Bile acid synthesis in the liver converts cholesterol to bile acids through the classic and alternative pathways. (A) Bile acid synthesis. The classic pathway involves 17 enzymes located in the endoplasmic reticulum, cytosol, mitochondria, and peroxisomes. Four CYP monooxygenases (CYP7A1, CYP8B1, CYP27A1 and CYP7B) are involved in hydroxylation reactions. In the classic pathway, a steroid nucleus undergoes hydroxylation, isomerization, and epimerization first, then oxidative cleavage of the side chain by CYP27A1, and cleavage of the 3-C side chain by peroxisomal β oxidation. CYP7A1 is the first and rate-limiting enzyme in the classic pathway, which synthesizes two primary bile acids (CA and CDCA) in the human liver. CYP8B1 is required for synthesis of CA. CYP27A1 catalyzes side-chain oxidation reactions. The alternative pathway is initiated by CYP27A1, followed by CYP7B1, and then further metabolized as the classic pathway to produce CDCA. (B) Conjugation of bile acids. Amidation (BACS and BAAT), sulfation (SULT2A1), and glucuronidation (UGT1A1, 2B4, and 2B7) at specific positions by specific enzymes are shown. (C) Biotransformation of bile acids. Secondary bile acids CDCA and LCA can be converted to more hydrophilic bile acids in the hepatocytes and intestine. Hydroxylation (by human CYP3A4 or mouse Cyp3a11), epimerization, and dehydroxylation interconverts CDCA to UDCA to LCA as well as other bile acids.