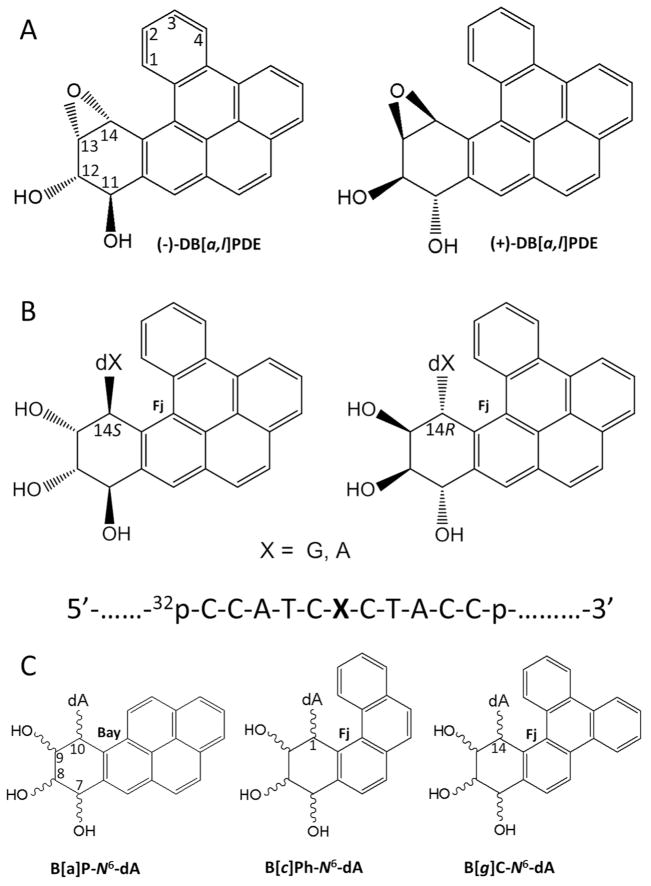
Figure 1.
(A) Structures and absolute configurations of the (−)- and (+)-anti-DB[a,l]PDE enantiomers that react with adenine or guanine in DNA. The “+” and “−” signs indicate the optical rotations of the two anti-DB[a,l]PDE enantiomer precursors. (B) Structures and configurations of the stereoisomeric S and R DB[a,l]P-dA or –dG adducts (X = A, or G); the sequence context of the 11-mer oligonucleotide sequence embedded in the double-stranded 135-mer oligonucleotide substrates is also shown. (C) Definitions of the bay regions of the stereoisomeric B[a]P-N6-dA adducts derived from (+)- or (−)B[a]PDE, and the fjord (Fj) regions of the stereoisomeric R and S adducts derived from the fjord region diol epoxides of benzo[c]phenanthrene (B[c]Ph) and benzo[g]chrysene (B[g]C) with absolute configurations analogous to those depicted in (A).