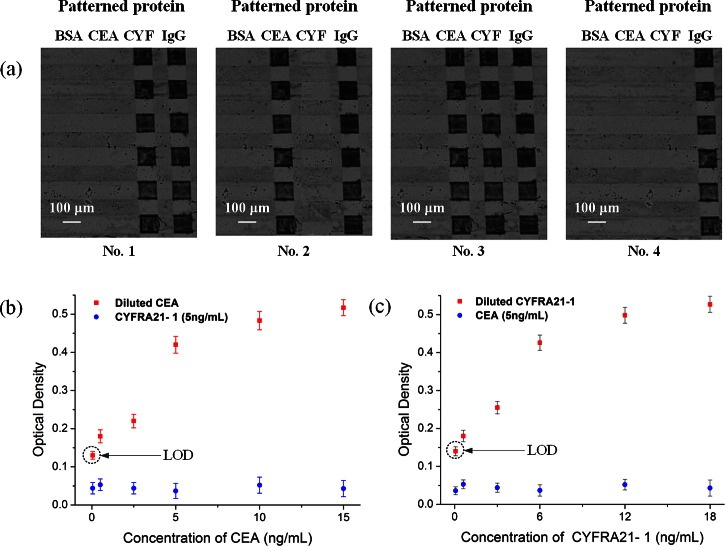
Figure 6.
Whole blood sample-to-answer assay using the proposed microfluidic devices: (a) Images of silver-enhanced signals on detection sites coated with anti-CEA antibody, anti-CYFRA21-1 antibody, anti-goat IgG as a positive reference and BSA as a negative reference. No. 1: sample containing only CYFRA21-1 antigen, No. 2: sample containing only CEA antigen, No. 3: sample containing both CEA and CYFRA21-1 antigens, No. 4: negative sample (no antigen). (b) The optical density obtained for immunoassays done with the mixture of CEA and CYFRA21-1 on the proposed chip, while keeping the concentration of CYFRA21-1 in constant (5 ng/ml) and varying the concentration of CEA (50 pg/ml, 500 pg/ml, 2.5 ng/ml, 5 ng/ml, 10 ng/ml, and 15 ng/ml). (c) The optical density obtained for immunoassays done with the mixture of CEA and CYFRA21-1 on the proposed chip, while keeping the concentration of CEA in constant (5 ng/ml) and varying the concentration of CYFRA21-1 (60 pg/ml, 600 pg/ml, 3 ng/ml, 6 ng/ml, 12 ng/ml, and 18 ng/ml). Error bars are based on standard deviation with n = 3.