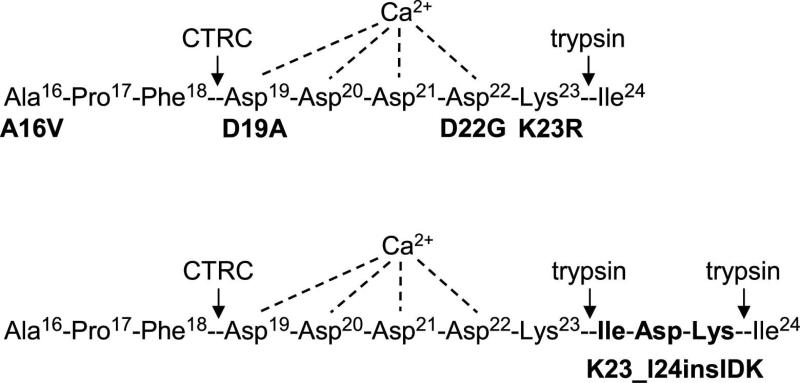
Figure 1.
Pancreatitis-associated mutations in the activation peptide of human cationic trypsinogen. Proteolytic cleavage sites for trypsin and chymotrypsin C (CTRC) and the putative Ca2+ binding site are also indicated. See text for details. Note that the N-terminal amino-acid of mature trypsinogen is Ala16, as the 15 amino-acid long secretory signal peptide is removed in the endoplasmic reticulum. Properties of the A16V mutant were published recently and it was not included in the present study [3, 5].