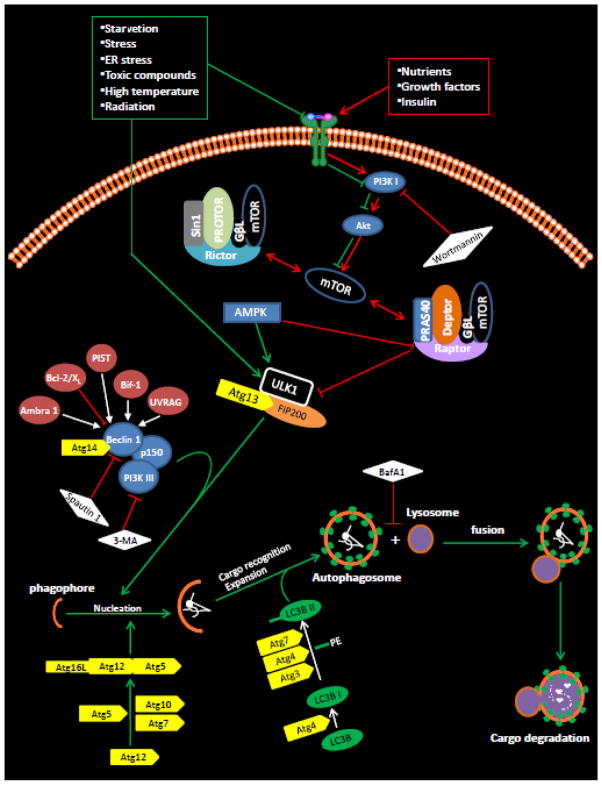
Figure 1. Autophagy in mammalian cells.
In the presence of amino acids, growth factors and ample energy, phosphorylated Ser-2448 of the mammalian target of rapamycin (mTOR) by PKB (Akt) [68, 69] inhibits autophagy via Ser-757 phosphorylation in ULK1 [70]. The phosphorylation and activation of mTOR simultaneously induce growth and proliferative responses. In contrast, in the absence of amino acids and growth factors or during starvation (i.e., increase in the AMP-to-ATP ratio) or during oxidative stress, phosphorylation of ULK1 at Ser-317, -467, -555, -637, -777 or Thr-574 is associated with ULK1 activation and dissociation of the raptor complex from the ULK1 complex leading to autophagy activation [70, 71]. In addition to phosphorylating ULK1, AMPK directly phosphorylates the mTOR binding partner raptor on two well-conserved serine residues, Ser-722/Ser-792, and this phosphorylation induces 14-3-3 binding with raptor. The phosphorylation of raptor by AMPK is required for the inhibition of mTORC1 and cell-cycle arrest induced by energy deprivation [72]. The phosphorylation of ULK1 and raptor on multiple residues ensures tight control of autophagy activation, and thus, prevents inapprorpiate activation of autophagy. It is suggested that ULK1 acts as a focal point for multiple signals that control autophagy [73], and it can bind to other autophagy-related (Atg) proteins. However, the mechanism that links the ULK1 complex to downstream complexes such as the PI3KIII complex during autophagy induction is not well known. Beclin 1 in the PI3KIII complex functions as a scaffold molecule that recruits other molecules, such as PIST, UVRAG and Bif-1, important for the nucleation or maturation of the phagophore into the autophagosome. The formation of the autophagosome relies on 2 ubiquitin-like conjugation systems. In the first, ATG7 and ATG10 catalyze the conjugation of ATG12 to ATG5 [2, 3]. ATG16L stabilizes the ATG12-ATG5 complex, which is required for the stimulation of the second conjugation reaction. At this time, cellular components primed for degradation are starting to be enclosed. The second conjugation involves 2 steps: first, ATG4 primes LC3 (ATG8) by exposing a glycine residue at the COOH-terminus [4], and, second, primed LC3 (or LC3-I) is then conjugated with the polar head of phosphatidylethanolamine (PE) to form LC3-PE (or LC3-II). This reaction is catalyzed by ATG7 and ATG3, and LC3-II is recruited to both the outer and inner faces of the growing autophagosome. Formation of LC3-II is used as a measure of autophagy induction because LC3-I is converted to LC3-II only during autophagy [74, 75]. The mature autophagosome then fuses with the lysosome leading to degradation of the contents of the autophagosome by lysosomal enzymes.