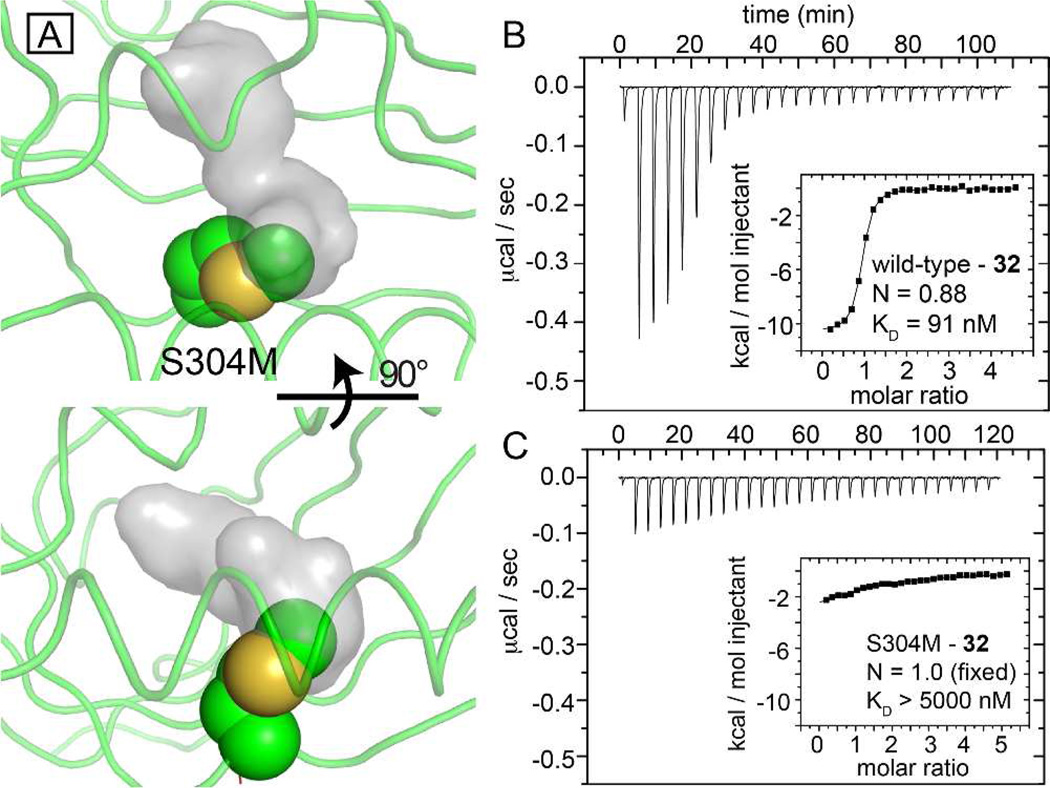
Figure 3.
A single internal mutation within the HIF-2α PAS-B cavity attenuates ligand binding. A) Two views of a model of the S304M mutation (spheres) suggests that the new sidechain will intrude upon the apo- protein cavity (grey surface, from PDB code: 3F1P).9b (B, C) ITC of wild-type (B) and S304M (C) complexes with compound 32, demonstrating that the mutation reduces the affinity of the protein over 50-fold, validating the biophysically-characterized ligand binding site.