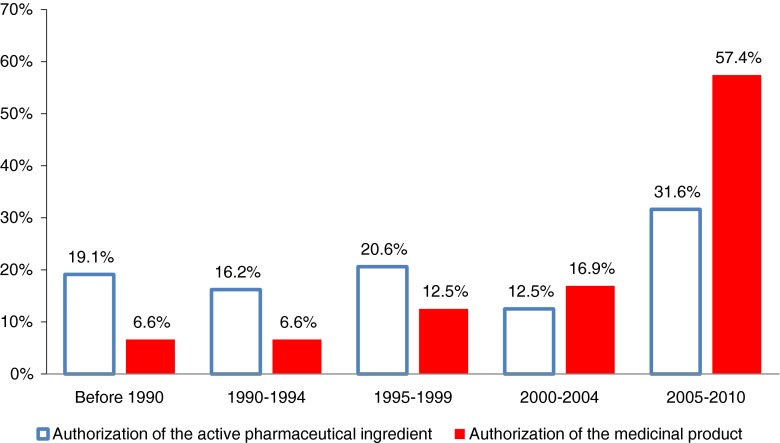
Fig. 3.
Year of authorization of the medicinal product and active pharmaceutical ingredient (API) [31]. For combination medicinal products the year the combination was authorized for the first time was chosen as the year of marketing authorization of the API. If several medicinal products containing the same API were investigated, the year the newest medicinal product was granted marketing authorization was chosen. If medicinal products containing different API were investigated, the year the newest medicinal product was granted marketing authorization was chosen as well as that of the corresponding API. For an API without determination of a medicinal product, the year the newest medicinal product with this API was authorized was chosen. For a therapeutic regimen with different API without determination of distinct medicinal products, the year the newest API was authorized was chosen, and the year of authorization of the medicinal product was determined to be the year of authorization of the corresponding API