Abstract
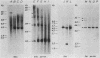
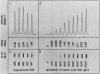
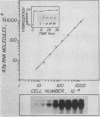
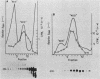
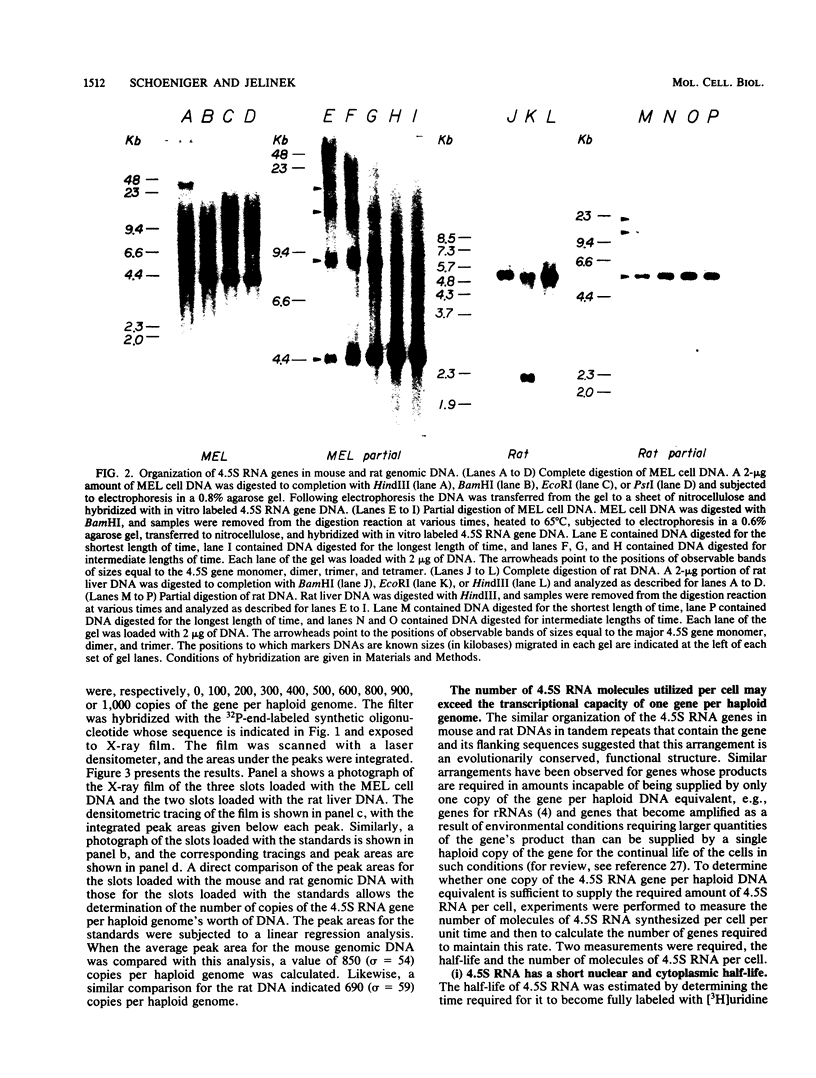
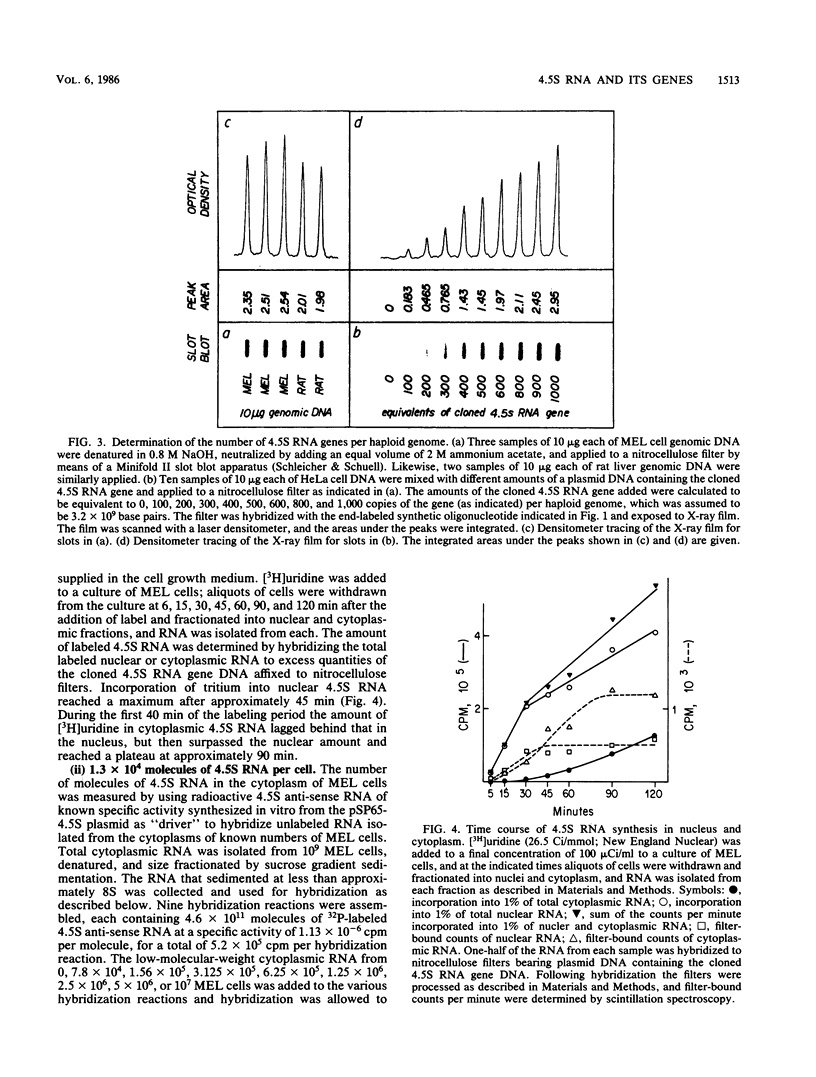
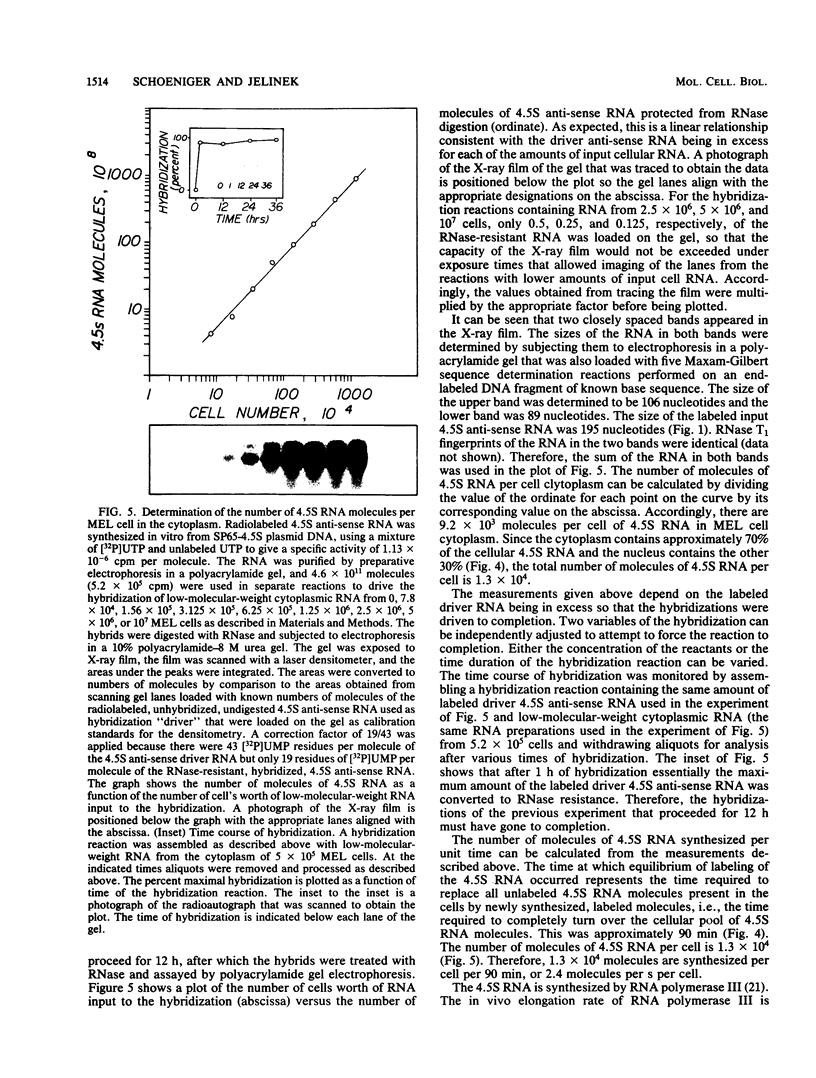
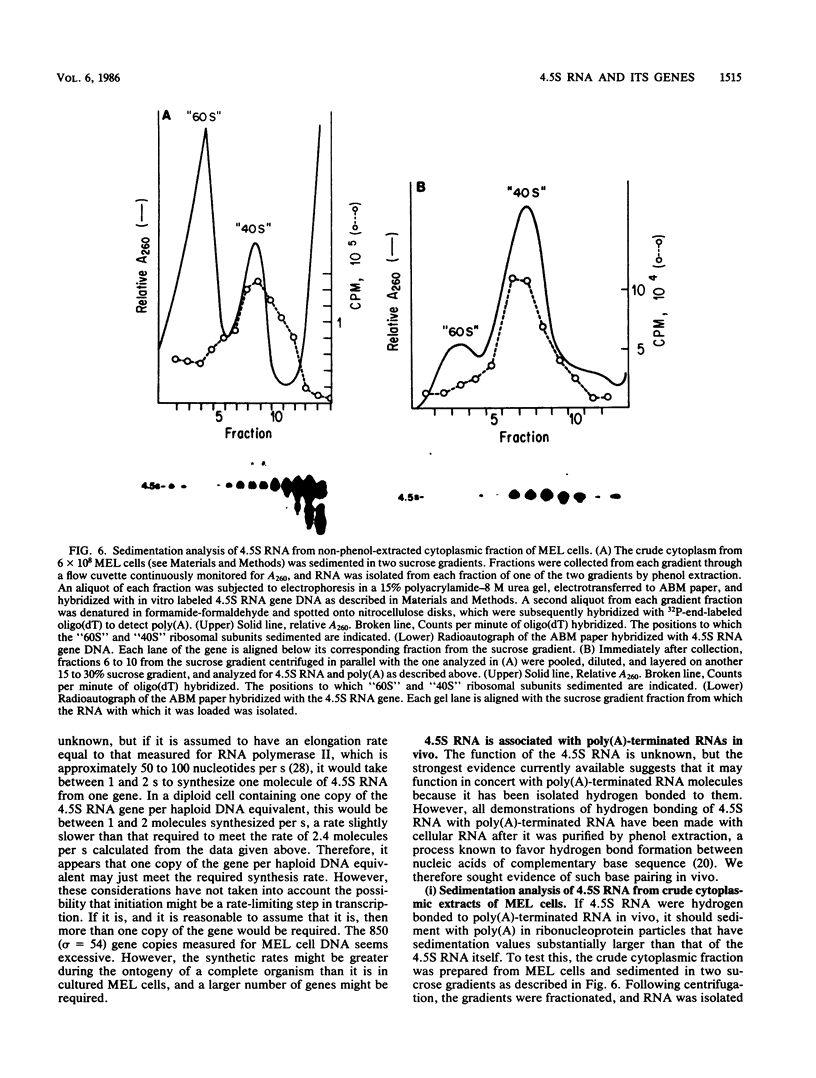
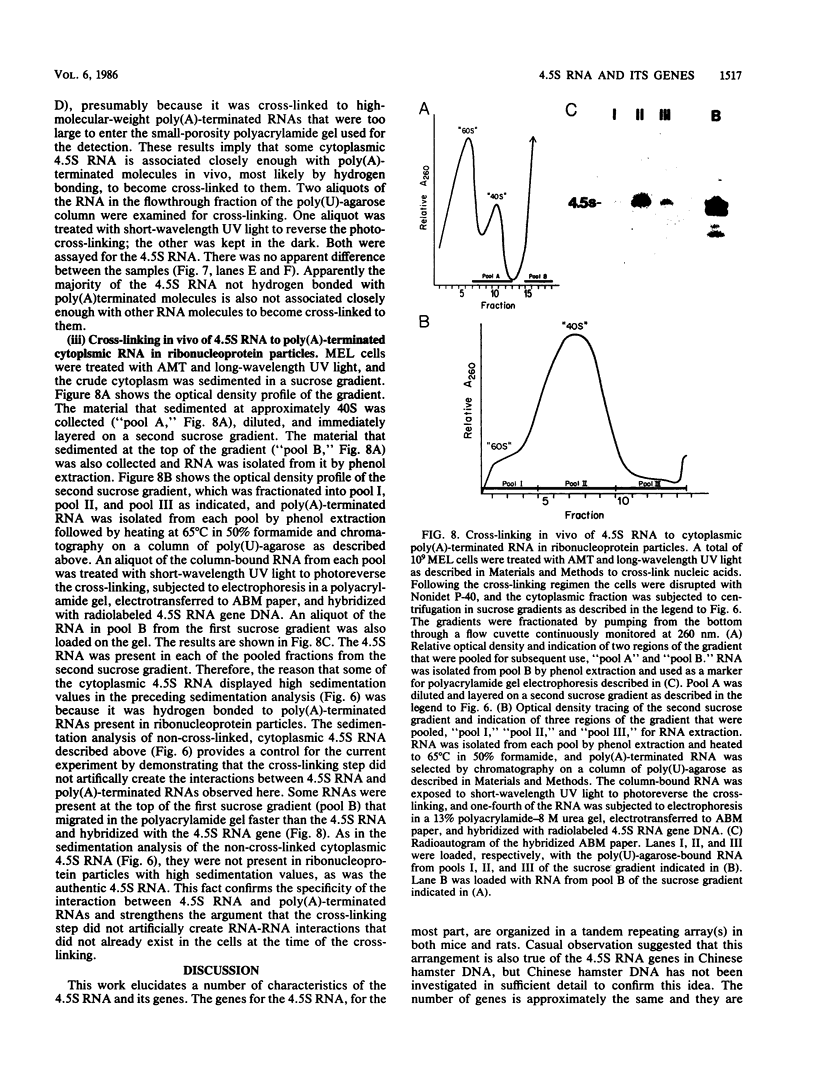
4.5S RNA is a group of RNAs 90 to 94 nucleotides long (length polymorphism due to a varying number of UMP residues at the 3' end) that form hydrogen bonds with poly(A)-terminated RNAs isolated from mouse, hamster, or rat cells (W. R. Jelinek and L. Leinwand, Cell 15:205-214, 1978; F. Harada, N. Kato, and H.-O. Hoshino, Nucleic Acids Res. 7:909-917, 1979). We have cloned a gene that encodes the 4.5S RNA. It is repeated 850 (sigma = 54) times per haploid mouse genome and 690 (sigma = 59) times per haploid rat genome. Most, if not all, of the repeats in both species are arrayed in tandem. The repeat unit is 4,245 base pairs long in mouse DNA (the complete base sequence of one repeat unit is presented) and approximately 5,300 base pairs in rat DNA. This accounts for approximately 3 X 10(6) base pairs of genomic DNA in each species, or 0.1% of the genome. Cultured murine erythroleukemia cells contain 13,000 molecules per cell of the 4.5S RNA, which can be labeled to equilibrium in 90 min by [3H]uridine added to the culture medium. The 4.5S RNA, therefore, has a short half-life. The 4.5S RNA can be cross-linked in vivo by 4'-aminomethyl-4,5',8-trimethylpsoralen to murine erythroleukemia cell poly(A)-terminated cytoplasmic RNA contained in ribonucleoprotein particles.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachellerie J. P., Hearst J. E. Specificity of the photoreaction of 4'-(hydroxymethyl)-4,5',8-trimethylpsoralen with ribonucleic acid. Identification of reactive sites in Escherichia coli phenylalanine-accepting transfer ribonucleic acid. Biochemistry. 1982 Mar 16;21(6):1357–1363. doi: 10.1021/bi00535a039. [DOI] [PubMed] [Google Scholar]
- Bachellerie J. P., Thompson J. F., Wegnez M. R., Hearst J. E. Identification of the modified nucleotides produced by covalent photoaddition of hydroxymethyltrimethylpsoralen to RNA. Nucleic Acids Res. 1981 May 11;9(9):2207–2222. doi: 10.1093/nar/9.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Weber C. S. Gene linkage by RNA-DNA hybridization. II. Arrangement of the redundant gene sequences for 28 s and 18 s ribosomal RNA. J Mol Biol. 1968 Jun 28;34(3):681–697. doi: 10.1016/0022-2836(68)90189-7. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Base-pairing interactions between small nuclear RNAs and nuclear RNA precursors as revealed by psoralen cross-linking in vivo. Cell. 1981 Nov;26(3 Pt 1):363–370. doi: 10.1016/0092-8674(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flach G., Johnson M. H., Braude P. R., Taylor R. A., Bolton V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kato N., Hoshino H. Series of 4.5S RNAs associated with poly(A)-containing RNAs of rodent cells. Nucleic Acids Res. 1979 Oct 25;7(4):909–917. doi: 10.1093/nar/7.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Toomey T. P., Leinwand L., Jelinek W. R. The Chinese hamster Alu-equivalent sequence: a conserved highly repetitious, interspersed deoxyribonucleic acid sequence in mammals has a structure suggestive of a transposable element. Mol Cell Biol. 1981 Jul;1(7):573–583. doi: 10.1128/mcb.1.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Jelinek W. R., Bachvarova R. Repetitive sequence transcripts and U1 RNA in mouse oocytes and eggs. Dev Biol. 1985 May;109(1):15–24. doi: 10.1016/0012-1606(85)90341-0. [DOI] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Leinwand L. A., Wydro R. M., Nadal-Ginard B. Small RNA molecules related to the Alu family of repetitive DNA sequences. Mol Cell Biol. 1982 Nov;2(11):1320–1330. doi: 10.1128/mcb.2.11.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J Biol Chem. 1984 Feb 10;259(3):2013–2021. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Richards O. C., Martin S. C., Jense H. G., Ehrenfeld E. Structure of poliovirus replicative intermediate RNA. Electron microscope analysis of RNA cross-linked in vivo with psoralen derivative. J Mol Biol. 1984 Mar 5;173(3):325–340. doi: 10.1016/0022-2836(84)90124-4. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Derman E., Molloy G. R., Tamm I., Darnell J. E. 5,6-Dichloro-1-Beta-D-ribofuranosylbenzimidazole inhibits initiation of nuclear heterogeneous RNA chains in HeLa cells. Science. 1976 Oct 22;194(4263):431–433. doi: 10.1126/science.982026. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]