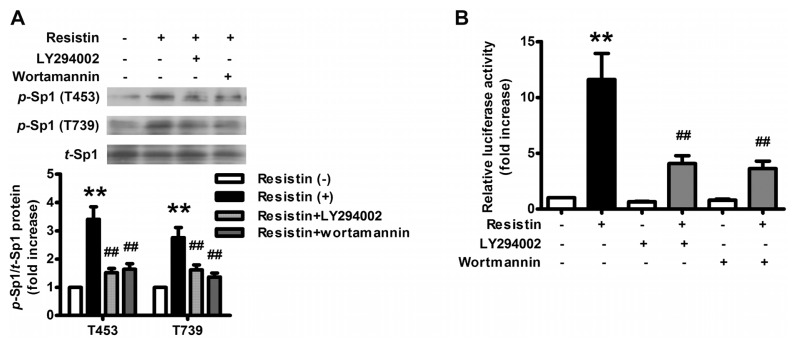
Figure 4.
Activated PI3K/Akt upon resistin interacted with Sp1 and activated it through phosphorylation. (A) Blockage of resistin-induced Sp1 activation by LY294002 and wortmannin in HO-8910 cells. Cells were pretreated with (+) or without (−) LY294002 (20 μM) and wortmannin (2 μM) for 1 h, then incubated in the presence (+) or in the absence (−) of resistin 100 ng/mL for 2 h. The top panel is a representative blot obtained from three independent experiments. The lower panel represents the summary results of Sp1 phosphorylation at either Thr-453 or Thr-739. Relative phosphorylated Sp1 protein levels were normalized to that of untreated cells; (B) Attenuation of resistin-induced Sp1 binding activity by LY294002 and wortmannin in HO-8910 cells. Cells were transfected with pSp1-luciferase reporter together with control plasmid. After being pretreated with (+) or without (−) LY294002 (20 μM) and wortmannin (2 μM) for 1 h, cells were incubated in the presence (+) or in the absence (−) of resistin 100 ng/mL for 24 h. Data are mean ± SEM from four independent triplicated experiments. The basal binding activity of each test plasmid is indicated as luciferase activity normalized by each internal control activity (PRL-TK). Relative luciferase activity was normalized with respect to the activity in untreated cells. **p < 0.01 vs. untreated cells, ##p < 0.01 vs. resistin treatment alone.