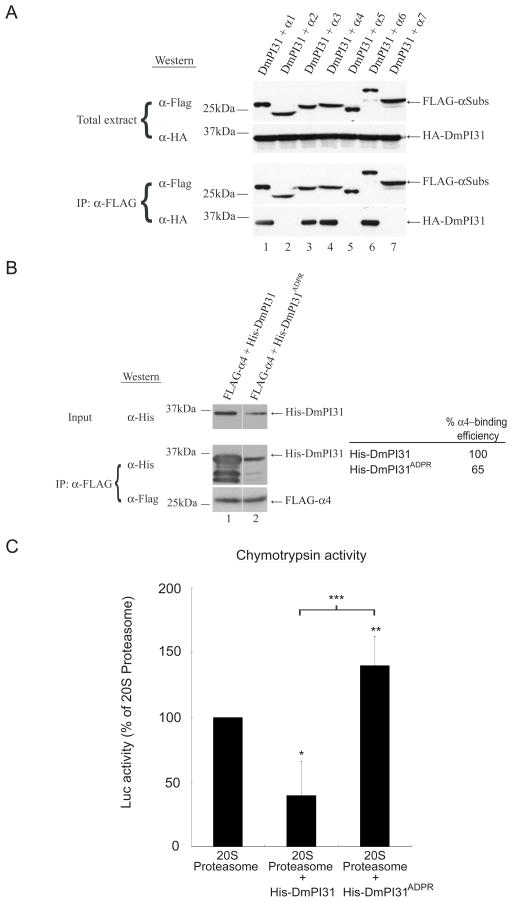
Figure 4. ADP-ribosylation relieves 20S inhibition by PI31.
(A) DmPI31 interacts with 20S proteasomes via select α-subunits: α1, α3, α4 and α6. (B) ADP-ribosylation significantly decreases the affinity of DmPI31 for α4. FLAG- α4 expressing 293 cell extract was incubated with modified DmPI31 (His-DmPI31ADPR) to test its effect on the α4:DmPI31 complex. Chemoluminescence was quantitated using ImageQuant LAS-4000 (GE Healthcare). α4-binding is reported as relative amount of immunoprecipitated His-DmPI31 compared to the level of input His-DmPI31. (C) Modification of DmPI31 relieves its inhibitory effect on 20S proteasomes. Purified bovine 20S proteasomes (0.1 μg) were incubated with His-DmPI31 and His-DmPI31ADPR proteins (2 μg each), and chymotrypsin-like proteasome activity was measured. Data are presented as mean ± standard deviation from three independent experiments. The value obtained for 20S proteasome was set as 100%. *, p=0.04, **, p=0.6 and ***, p=0.045. Statistical analysis was performed with a two-tailed, paired, t-test.