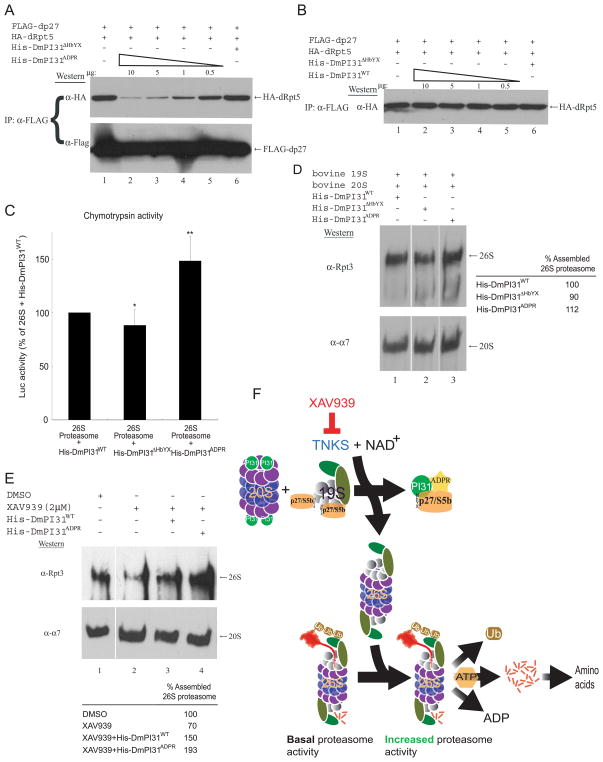
Figure 7. ADP-ribosylation increases DmPI31 affinity for dp27 to regulate proteasome assembly, and model for proteasome regulation by ADP-ribosylation.
(A and B) ADP-ribosylation increased the affinity of DmPI31 for dp27, allowing it to compete with dRpt5 for chaperones in a dose-dependent manner. Anti-FLAG IP using 293 cell extracts standardized for FLAG-dp27 and HA-dRpt5 expressions were supplemented with in vitro modified (A) and unmodified (B) DmPI31 to assess their ability to compete with dRpt5 for dp27. (C) ADP-ribosylated DmPI31 activated 26S proteasome activity in vitro to levels beyond that seen for its unmodified control. Purified bovine 26S proteasome (0.1μg) was incubated with His-DmPI31, His-DmPI31ΔHbYX and His-DmPI31ADPR (2 μg each), and chymotrypsin-like proteasome activity was assayed. Data are presented as mean ± standard deviation from three independent experiments. The value obtained for “26S + His-DmPI31WT” was set as 100%. *, p=0.46 and **, p=0.007. Statistical analysis was performed with a two-tailed, paired, t-test. (D) DmPI31 modification promotes de novo assembly of the 26S proteasome. Purified bovine 19S and 20S proteasome particles (1 μg each) were incubated with His-DmPI31, His-DmPI31ΔHbYX and His-DmPI31ADPR (5 μg each) in the presence of ATP, followed by native gel analysis to assess the effect of modified DmPI31 on proteasome assembly. (E) Modified DmPI31 rescues XAV939-induced defects in 26S proteasome assembly to a greater extent than its unmodified control. 200 μg of embryo extracts treated with the TNKS-inhibitor were supplemented with 5 μg of respective proteins followed by native gel analysis. (D and E) Native gels were analyzed via Western blot using anti-Rpt3 (for 26S proteasome) and anti- α7 (for 20S proteasome). Chemoluminescence was quantitated using ImageQuant LAS-4000 (GE Healthcare). 26S proteasome assembly status is reported as relative intensity of Rpt3 bands. (F) Model for proteasome regulation by ADP-ribosylation. PI31 is a conserved proteasome regulatory protein that is required for normal 26S proteasome activity in vivo and organismal viability (Bader et al., 2011). ADP-ribosylation of PI31 by TNKS, which transfers the ADP-ribose group from NAD+, activates PI31 and leads to increased proteolytic activity of the 26S proteasome by promoting its assembly. Specifically, ADP-ribosylation of PI31 causes decreased affinity for 20S proteasome α-subunits and dislodges PI31 from 20S particles. On the other hand, ADP-ribosylation increases binding of PI31 to the assembly chaperones dp27 and dS5b and thereby sequesters them away from 19S regulatory particles. Taken together, this promotes 26S proteasome assembly and facilitates the breakdown of intracellular proteins. Inhibition of TNKS by small-molecule compounds, such as XAV939, blocks this process and reduces 26S proteasome activity. Therefore, TNKS-inhibitors represent a novel class of proteasome inhibitors that target the assembly of 26S proteasomes. See also Figure S6.