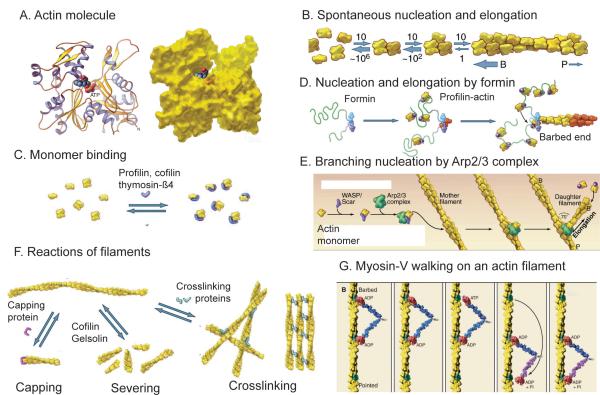
Figure 2.
Structures of actin and diagrams of fundamental reactions. A, Ribbon and space filling models of the actin molecule (pdb:1ATN). B, Spontaneous nucleation and elongation. Dimers and trimers are unstable. Longer polymers grow rapidly at the barbed end (B) and slowly at the pointed end (P). C, Actin monomer binding proteins. Thymosin-ß1 blocks all assembly reactions; profilin promotes nucleotide exchange and inhibits pointed end elongation and nucleation but not barbed end elongation; cofilin inhibits nucleotide exchange and promotes nucleation. D, Nucleation and elongation by formins. Formins initiate polymerization from free actin monomers and remain associated with the growing barbed end. Profilin-actin binds to formin and transfers actin onto the barbed end of the filament. E, Nucleation by Arp2/3 complex. Nucleation promoting factors such as WASp bind an actin monomer and Arp2/3 complex. Binding to the side of a filament completes activation, and the barbed end of the daughter filament grows from Arp2/3 complex. F, Reactions of actin filaments. Capping proteins bind to and block barbed ends; cofilin and gelsolin sever filaments; crosslinking proteins assemble networks and bundles of actin filaments. G, Myosin motors, such as myosin-V, use cycles of ATP hydrolysis to walk along actin filaments, generally toward the barbed end. (Redrawn from images in T.D. Pollard and W.C. Earnshaw, Cell Biology, W.B. Saunders, 2007.)