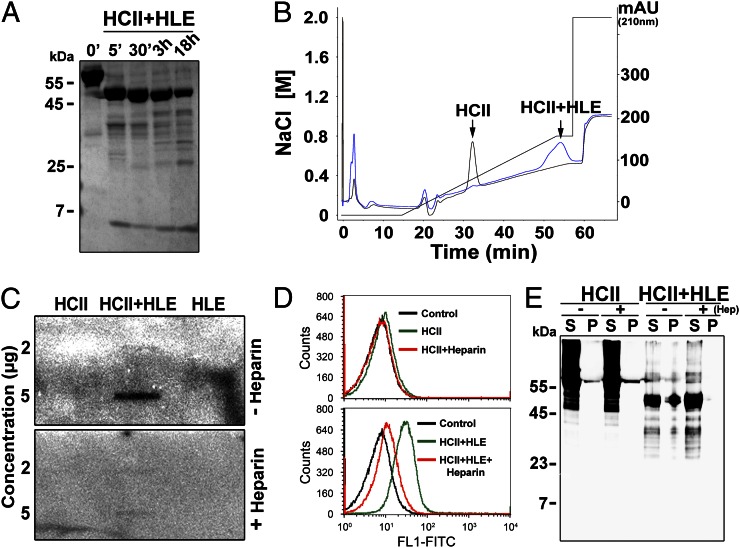
FIGURE 1.
Proteolytically cleaved HCII binds to LPS and bacteria. (A) SDS-PAGE analysis of HCII subjected to HLE. Incubation times are indicated. (B) Comparison of heparin binding of HCII (black) and HLE-digested HCII (blue) using heparin affinity chromatography (fast protein liquid chromatography). (C) Slot blot analysis for detection of binding of HCII, HCIIa (HCII+HLE), and HLE to 125I-labeled LPS with or without heparin. (D and E) Analyses of interaction between HCII or HLE-cleaved HCII and E. coli bacteria in presence or absence of heparin. (D) The interaction between E. coli and intact and cleaved HCII was analyzed by flow cytometry. Top panel: bacteria incubated with intact HCII; bottom panel: bacteria incubated with cleaved HCII (HCII+HLE). Heparin blocked the binding of HCIa to the bacteria (bottom panel). Representative histograms are shown (n = 3; Control: E. coli only). (E) Evaluation of binding of intact and cleaved HCII to E. coli using pulldown assays. A representative Western blot is shown (n = 3). Heparin (Hep) abolished binding of HCIIa (HCII+HLE) to the pellet (P+Hep, rightmost lane). mAU, Milli-absorbance unit; P, bacterial pellet; S, supernatant.