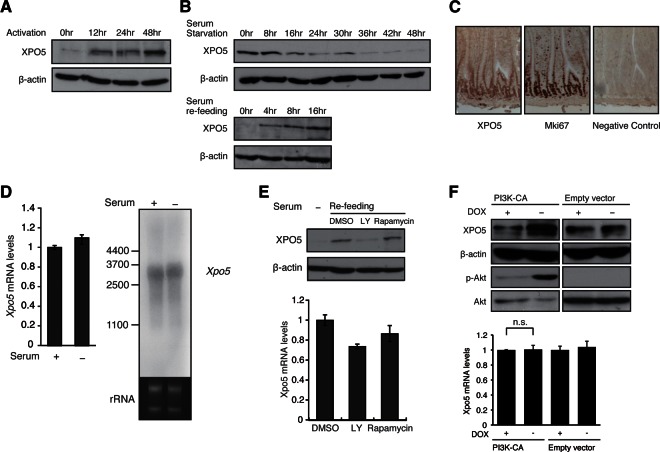
FIGURE 2.
XPO5 is rapidly induced during cell cycle entry and is dependent on a PI3K-mediated post-transcriptional mechanism. (A) Western blot for XPO5 at the indicated time points after in vitro activation of T-cell samples as detailed in the Supplementary Materials and Methods. Reblotting for β-actin is a loading control. (B) Western blot for XPO5 at indicated time points after serum starvation or refeeding of MEF cells. (C) Immunohistochemical analysis in the sections of small intestine stained with antibodies against XPO5 and proliferation marker Mki67. Note the costaining of XPO5 and Mki67 in the same cell population, indicating that XPO5 protein is preferentially expressed in the proliferating cells. (D) RNA level of Xpo5 in serum-fed and -starved NIH3T3 cells was determined by real-time PCR and Northern blotting. β-Actin and rRNA were measured as the internal controls. (E) Western blot for XPO5 in MEF cells treated with dimethyl sulfoxide (DMSO), LY-294002, and Rapamycin during cell cycle entry. The inhibitors were added in the culture 2 h before serum refeeding. RNA levels of Xpo5 were determined by real-time PCR using the same samples. β-Actin served as the loading control. (F) XPO5 induction following PI3K activation. Doxycycline (DOX)- or DMSO-treated PI3K constitutively active (CA) and negative control Tet-off NIH3T3 lines were subjected to Western blotting for XPO5, β-actin, phosphorylated AKT, and AKT. RNA levels of Xpo5 were determined by real-time PCR using DOX- or DMSO-treated PI3K CA and negative control Tet-off NIH3T3 lines.