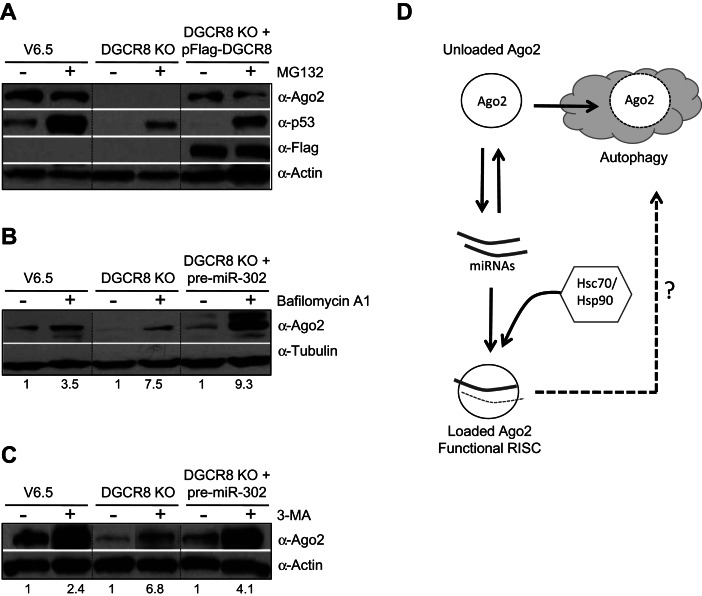
FIGURE 4.
Inhibition of the autophagy stabilizes unloaded Ago2 protein. (A) V6.5, DGCR8 KO, and DGCR8 KO ESCs previously transfected with pFlag-DGCR8 for 24 h were treated with MG132 (see Materials and Methods). Ago2, Flag-DGCR8, and p53 levels in total cell extracts were analyzed by Western blot. Actin serves as loading control. (B,C) V6.5 and DGCR8 KO ESCs were treated with the lysosomal inhibitor Bafilomycin A1 (B) and the inhibitor of autophagosome formation 3-MA (C) (see Materials and Methods). Ago2 levels in total cell extracts were analyzed by Western blot. Tubulin or Actin serves as loading control. The numbers on the bottom of Western blots indicate the relative Ago2 abundance (normalized to loading control). For each cell-type the relative Ago2 levels in the untreated lane is set to 1.0. (D) Model: Ago2 protein levels are post-transcriptionally coupled to miRNA levels. The Hsc70/Hsp90 chaperone complex aids in the loading of miRNAs into Ago2. Unloaded Ago2 is degraded—a process that involves cellular autophagy. The involvement of autophagy and/or other pathways in the turnover of loaded Ago2 still remains unclear.