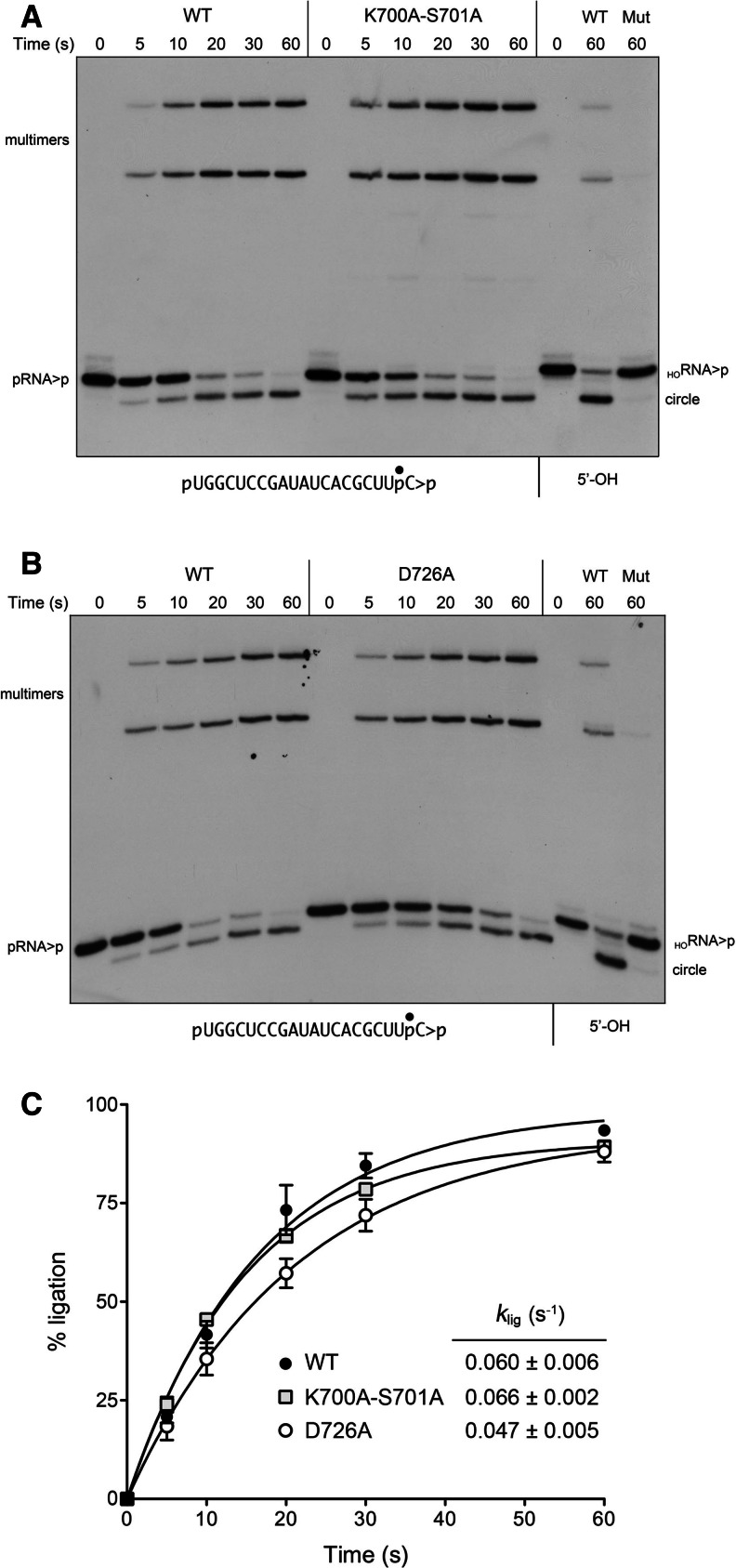
FIGURE 5.
A 5′-PO4 bypasses inactivating mutations of the kinase domain. (A,B) Reaction mixtures containing 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, 10 mM MgCl2, 0.1 mM ATP, 2 mM DTT, 20 nM pRNA>p substrate (as shown), and 1 µM wild-type AtRNL (A,B) or mutants K700A-S701A (A) or D726A (B) as specified were incubated at 22°C. The reactions were quenched at the times indicated, and the products were analyzed by urea-PAGE. Control reactions containing 20 nM HORNA>p substrate (5′-OH) and 1 µM wild-type (WT) or mutant AtRNLs were incubated for 60 sec and analyzed in parallel in the lanes at right. (C) The extents of pRNA>p ligation by WT, K700A-S701A, and D726A AtRNL are plotted as a function of reaction time. Each datum is the average of three (WT and K700A-S701A) or four (D726A) separate experiments ±SEM. Nonlinear regression curve fits of the data and the derived rate constants are shown.