FIGURE 7.
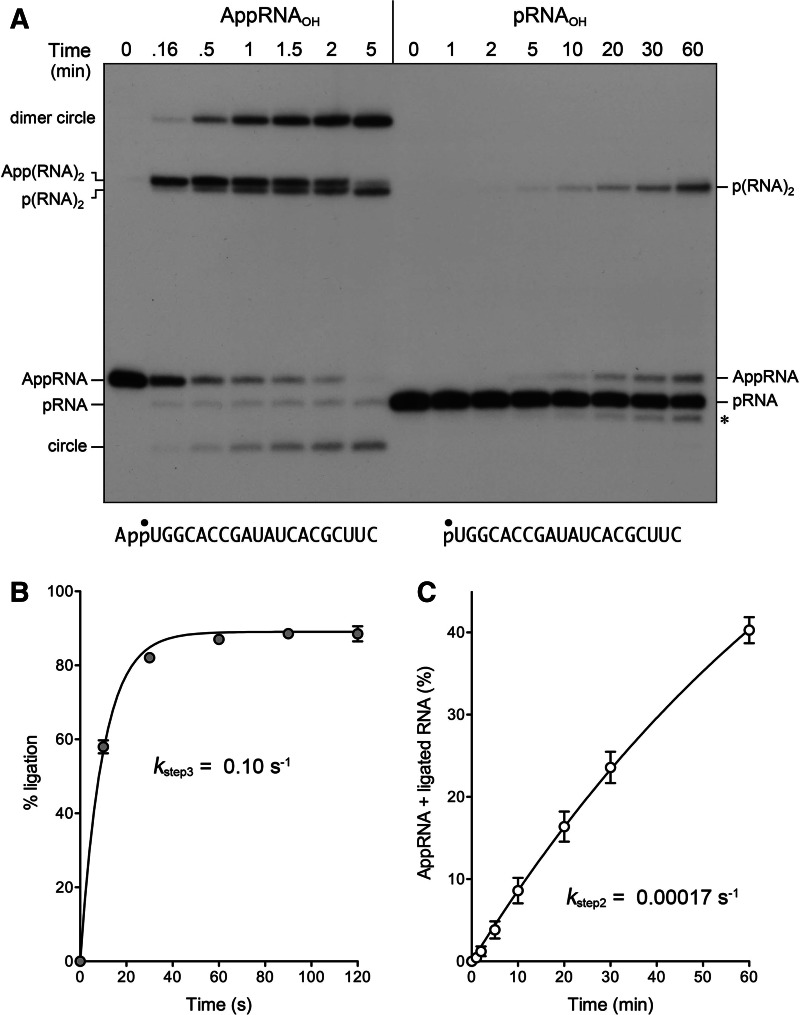
The 2′-PO4 requirement for sealing is enforced at the RNA 5′-adenylylation step. (A) AppRNAOH and pRNAOH ligation. Reaction mixtures containing 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, 10 mM MgCl2, 2 mM DTT, 20 nM 20-mer AppRNAOH, or pRNAOH substrate (shown at bottom with the 32P label denoted by •), 1 µM AtRNL, and either no added ATP (AppRNAOH reaction) or 0.1 mM ATP (pRNAOH reaction) were incubated at 22°C. The reactions were quenched with formamide, EDTA at the times specified, and the products were analyzed by urea-PAGE. The positions and identities of the radiolabeled substrates and products are indicated at left and right. (The asterisk at right denotes an n − 1 decay product derived from pRNAOH during prolonged incubation with AtRNL; the formation of this species, comprising 8% of the total labeled RNA at 60 min, was unaffected by omission of ATP but was eliminated by omission of Mg2+ [data not shown].) (B) AppRNAOH ligation kinetics. The extent of ligation is plotted as a function of reaction time. A nonlinear regression curve fit of the data (average of four experiments ±SEM) and the derived step 3 rate constant are shown. (C) pRNAOH reaction kinetics. The levels of AppRNA plus ligated RNA (as percent of total labeled RNA) are plotted as a function of reaction time. A nonlinear regression curve fit of the data (average of three experiments ±SEM) and the derived step 2 rate constant are shown.