Abstract
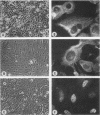
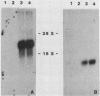
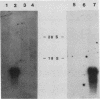
The goal of this work was to establish an assay for transformation of epithelial cells. Two epithelial cell lines were obtained after microinjecting transforming genes into primary rabbit mammary secretory cells. The cell lines were analyzed for their oncogenic potential and for the maintenance of a differentiated phenotype. A fully transformed cell line, which retained epithelial cell organization, was obtained by coinjecting simian virus 40 DNA and the activated human c-Ha-ras gene. The proliferation rate of these cells was high, with a doubling time of 16 h. Their growth was anchorage independent, and they had lost contact inhibition. The cells were tumorigenic in nude mice, but had no metastatic potential. Both microinjected DNAs were efficiently transcribed and translated, in contrast to the casein genes, which were expressed in primary cells but not in the transformed cell line. An immortalized cell line established after injection with simian virus 40 DNA alone was characterized by a moderate rate of proliferation with a doubling time of approximately 30 h. The growth of these cells was contact inhibited and anchorage dependent. The cells were not tumorigenic in nude mice. The viral DNA was expressed during early passages, as shown by the presence of the large T antigen in cell nuclei, but not at later passages. A high number of lactogenic hormone receptors were found associated with the cell surface. Despite the presence of these receptors, no induction of genes coding for milk proteins was observed after addition of prolactin. These data demonstrate that this assay system can be used to assess the immortalizing and transforming potential of candidate oncogenes in epithelial cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P. SV-40 INDUCED PROLIFERATION OF TISSUE CULTURE CELLS OF RABBIT, MOUSE, AND PORCINE ORIGIN. Proc Soc Exp Biol Med. 1963 Dec;114:721–727. doi: 10.3181/00379727-114-28780. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Wong C., Medina D. Transformation of mouse mammary epithelial cells by papovavirus SV40. Exp Mol Pathol. 1984 Feb;40(1):79–108. doi: 10.1016/0014-4800(84)90068-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- Danielson K. G., Oborn C. J., Durban E. M., Butel J. S., Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Microinjection of tissue culture cells. Methods Enzymol. 1983;101:482–492. doi: 10.1016/0076-6879(83)01033-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Suard Y. L., Bogenmann E., Reggio H., Racine L., Kraehenbuhl J. P. Effect of cell shape change on the function and differentiation of rabbit mammary cells in culture. J Cell Biol. 1983 May;96(5):1425–1434. doi: 10.1083/jcb.96.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Jaggi R., Kozma S. C., Ball R., Muellener D., Wetherall N. T., Davis B. W., Groner B. New acceptor cell for transfected genomic DNA: oncogene transfer into a mouse mammary epithelial cell line. Mol Cell Biol. 1985 Jan;5(1):268–272. doi: 10.1128/mcb.5.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl J. P. Dispersed mammary gland epithelial cells. I. Isolation and separation procedures. J Cell Biol. 1977 Feb;72(2):390–405. doi: 10.1083/jcb.72.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Neer A., Baran N., Manor H. In situ hybridization analysis of polyoma DNA replication in an inducible line of polyoma-transformed cells. Cell. 1977 May;11(1):65–71. doi: 10.1016/0092-8674(77)90317-8. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suard Y. M., Haeuptle M. T., Farinon E., Kraehenbuhl J. P. Cell proliferation and milk protein gene expression in rabbit mammary cell cultures. J Cell Biol. 1983 May;96(5):1435–1442. doi: 10.1083/jcb.96.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suard Y. M., Kraehenbuhl J. P., Aubert M. L. Dispersed mammary epithelial cells. Receptors of lactogenic hormones in virgin, pregnant, and lactating rabbits. J Biol Chem. 1979 Oct 25;254(20):10466–10475. [PubMed] [Google Scholar]
- Suard Y. M., Tosi M., Kraehenbuhl J. P. Characterization of the translation products of the major mRNA species from rabbit lactating mammary glands and construction of bacterial recombinants containing casein and alpha-lactalbumin complementary DNA. Biochem J. 1982 Jan 1;201(1):81–90. doi: 10.1042/bj2010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Sheffield J. B., Coutinho W. G. Murine mammary tumor virus (MuMTV) infection of an epithelial cell line established from C57BL/6 mouse mammary glands. Virology. 1978 Oct 1;90(1):12–22. doi: 10.1016/0042-6822(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Watkins J. F. The SV40 rescue problem. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):355–362. doi: 10.1101/sqb.1974.039.01.046. [DOI] [PubMed] [Google Scholar]