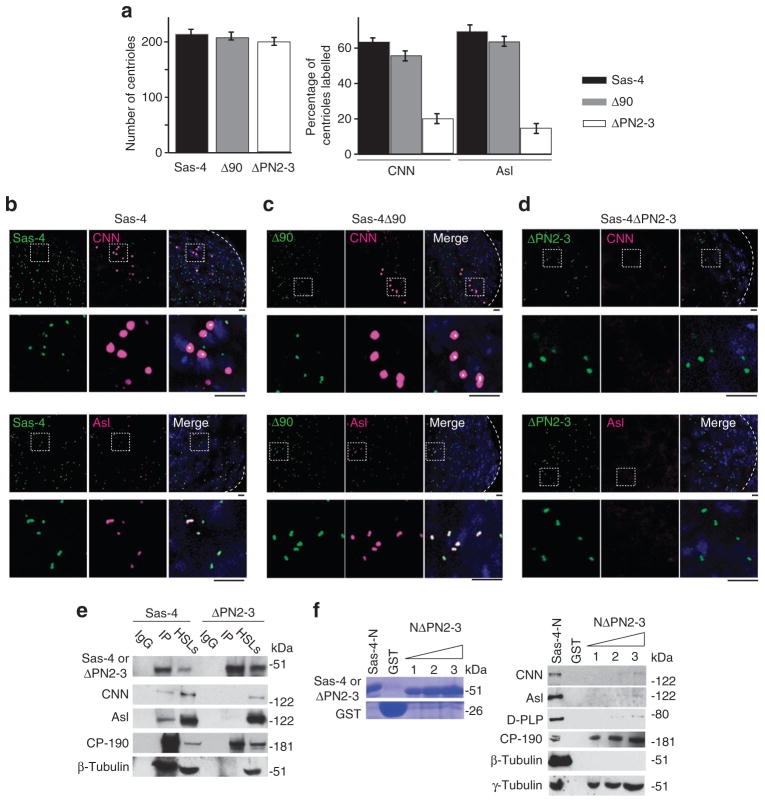
Figure 6. S-CAP complex components bind to centrosomes via the PN2-3 domain of Sas-4.
(a–d) In vivo, the PN2-3 domain is necessary for Sas-4’s recruitment of S-CAP complex components to a centriole. (a) The number of centrioles observed at the tip of a testis ( < 60 μm from the hub) and the fractions of centrioles that are positive for CNN or Asl. The mean ± s.e.m. of six independent testes are shown. (b, c) CNN and Asl are recruited to centrosomes of Sas-4s2214-null mutants that express Sas-4 or Sas-4Δ90. (d) In contrast, these proteins are not recruited to centrioles when Sas-4ΔPN2-3 is expressed. Dashed boxes mark the enlarged areas. Scale bar, 2 μm. (e) In vitro, the PN2-3 domain is necessary for Sas-4’s binding to S-CAP complex components and their recruitment to a centriole. Co-immunoprecipitation experiments using extracts of S2 cells transfected with Sas-4 or Sas-4ΔPN2-3. In cells that overexpress Sas-4, CNN, Asl, CP-190, β-tubulin and γ-tubulin are immunoprecipitated (IP) from high-speed cell lysates (HSLs). In contrast, CP-190 and γ-tubulin but not CNN, Asl and β-tubulin are immunoprecipitated from HSLs of S2 cells that overexpress Sas-4ΔPN2-3. IgG: beads coated with a mouse IgG. (f) The PN2-3 domain of Sas-4 is required to pull down Asl, CNN or β-tubulin from embryonic HSLs. The purified recombinant proteins used are shown in Coomassie-stained gels. Sas-4-N–GST (which only includes the first 190 amino acids of Sas-4) pulls down Asl, CNN, β-tubulin, CP-190 and γ-tubulin from embryonic lysates. However, a Sas-4-N–GST variant, which lacks its PN2-3 domain (‘NΔPN2-3’), can only pull down CP-190 and γ-tubulin.