Figure 5.
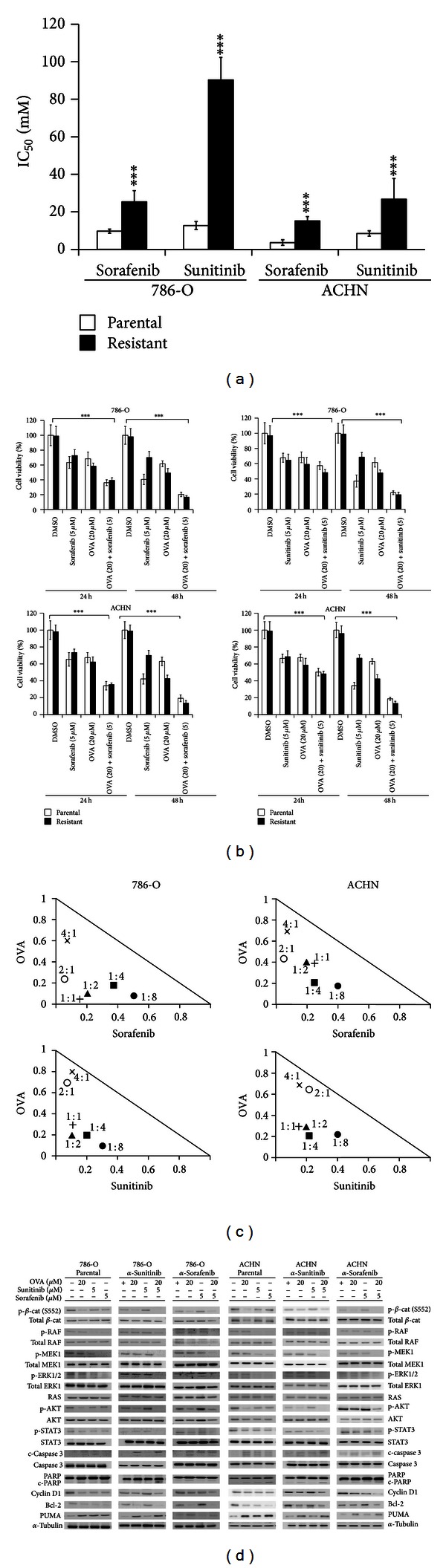
Ovatodiolide in combination therapy and against TKI-resistant RCC cell lines. (a) The half maximal inhibitory concentration of the conventional chemotherapy drugs sorafenib and sunitinib in RCC cells. Data are mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001. (b) Cell viability assay of both parental and TKI-resistant 786-O and ACHN cells treated with 20 μM ovatodiolide, 5 μM sorafenib or sunitinib, or their combination for 24 and 48 hr. Data are mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001. (c) Isobologram analysis of the combination of ovatodiolide and sorafenib or sunitinib. Symbols designate the combination index value for each fraction affected. The curves were generated by the use of CalcuSyn software to fit the experimental points. Data are representative of 3 independent experiments. Values below the line are synergistic, those close to the line are additive, and those above the line are antagonistic. (d) Western blot analysis of the combined effect of ovatodiolide (20 μM) with sorafenib (5 μM) or sunitinib (5 μM) for 48 hr comparing the parental, anti-sunitinib (α-sunitinib), and anti-sorafenib (α-sorafenib) 786-O or ACHN cells. Evaluation included TKIs targeting RAS-RAF-MEK1-ERK1 signaling and pSTAT3 status and ovatodiolide-targeted p-β-catenin (S552). Cytotoxicity was compared by levels of apoptotic cleaved caspase 3 and PARP, antiapoptotic Bcl-2, and apoptotic PUMA and cell cycle cyclin D1.
