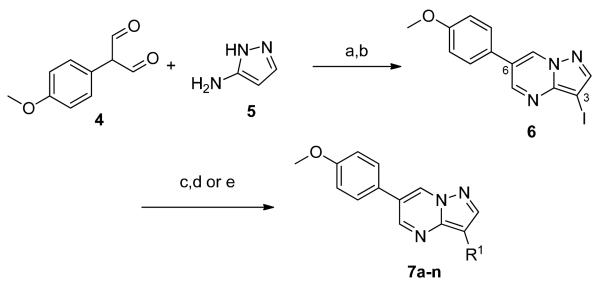
Scheme 1.
Reactions and conditions: (a) AcOH, EtOH, 170 °C, 10 min., μW, 92%; (b) NIS, DMF; (c) diboronpinacol ester, Pd(dppf)Cl2·DCM, KOAc, DMF, 100 °C, 16 h; (d) R1X, Pd(dppf)Cl2·DCM, K3PO4, 1,4-dioxane, H2O, 120 °C, 30 min., μW, 17–27% (3 steps); (e) R1B(OH)2, Pd(dppf)Cl2·DCM, K3PO4, 1,4-dioxane, H2O, 120 °C, 30 min., μW, 10-65% (2 steps).