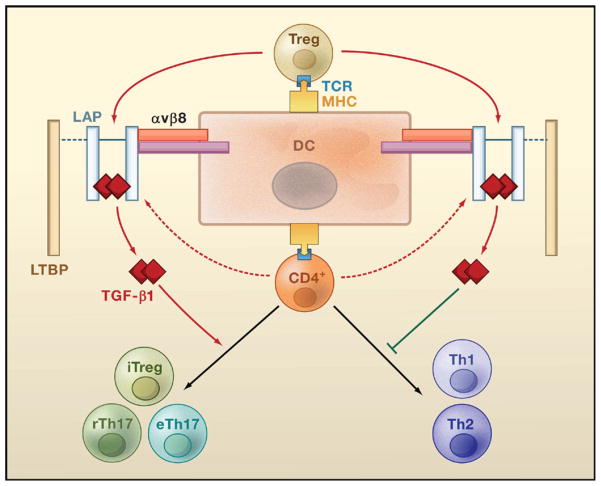
Figure 4. A Three-Cell Model for TGF-β1-Dependent Regulation of T Cells.
Upon recognition of antigens presented by dendritic cells (DCs), Treg cells are activated and secrete the latent form of TGF-β1. This latent form comprises a TGF-β1 dimer associated with the latency-associated protein (LAP), which may additionally recruit latent TGF-β-binding protein (LTBP). Interaction of LAP with αvβ8 integrin expressed by DCs triggers degradation of LAP (mediated by un-identified proteases, not shown) and the release of the active form of TGF-β1. Active TGF-β1 subsequently acts on naive CD4+ T cells via a paracrine mechanism to inhibit their differentiation into Th1 or Th2 cells and to promote their differentiation into induced regulatory T (iTreg) cells, regulatory Th17 (rTh17) cells, or effector Th17 (eTh17) cells. Activated CD4+ T cells can also produce low amounts of latent TGF-β1 that potentially regulate T cell differentiation through an autocrine route.