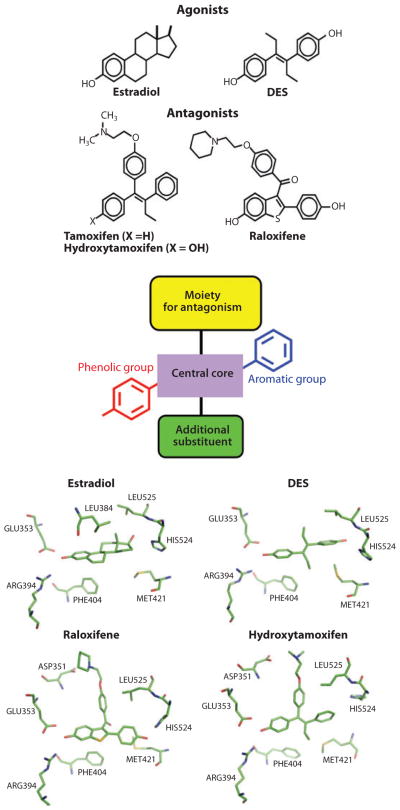
Figure 5.
Principles of ligand design as agonists and antagonists for the estrogen receptor (ER). Estrogens (agonists) include the natural ligand E2 and the synthetic hormone diethylstilbestrol (DES). Tamoxifen/hydroxytamoxifen and raloxifene are antiestrogens and function more as SERMs (selective estrogen receptor modulators). Although mimicking some general features of the basic molecular structure in these ligands, including the central core, with an aromatic and phenolic group on either sides, additional moieties such as extensions along one of the rings can convert ligands from agonists to antagonists on the basis of the protrusion of this group disrupting the agonist conformation of H12. Close-up views of the pocket around these four ligands are provided at the bottom, showing that the conserved features of their chemical frame are recognized similarly within the ER pocket (165–167).