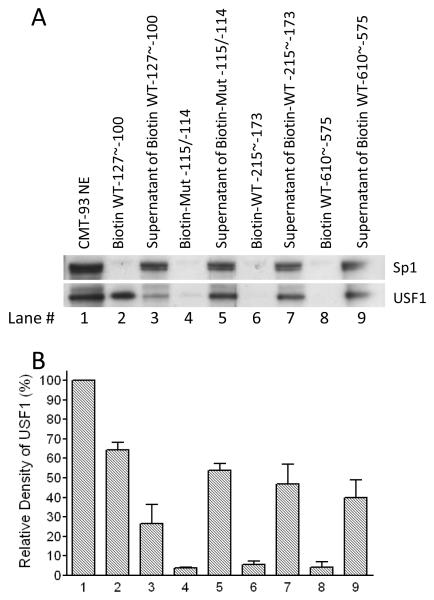
Figure 6.
Biotin affinity purification and western blot analysis. Dynabeads MyOne Streptavidin C1 beads were coated with double-stranded, 5′-biotinylated oligonucleotides and incubated with nuclear extracts from CMT-93 cells. After magnetic separation, the supernatants and the protein/biotinylated oligonucleotides/streptavidin complexes were analyzed by western blotting. USF1, but not Sp1 (included as a negative control) was captured on beads coated with the biotinylated oligonucleotide WT −127 to −100 (lane 2), largely clearing the supernatant (lane 3). Oligonucleotide Mut −115/−114 eliminated binding to USF1 (lane 4), so that almost all USF1 protein remained in the supernatant (lane 5). Similar results were observed using two other 5′-biotinylated oligonucleotides WT −215 to −173 and WT −610 to −575 that do not include any E-box motif (lane 6-9). Densitometry analysis was performed in four experiments and quantitative data are presented in Figure 6B.