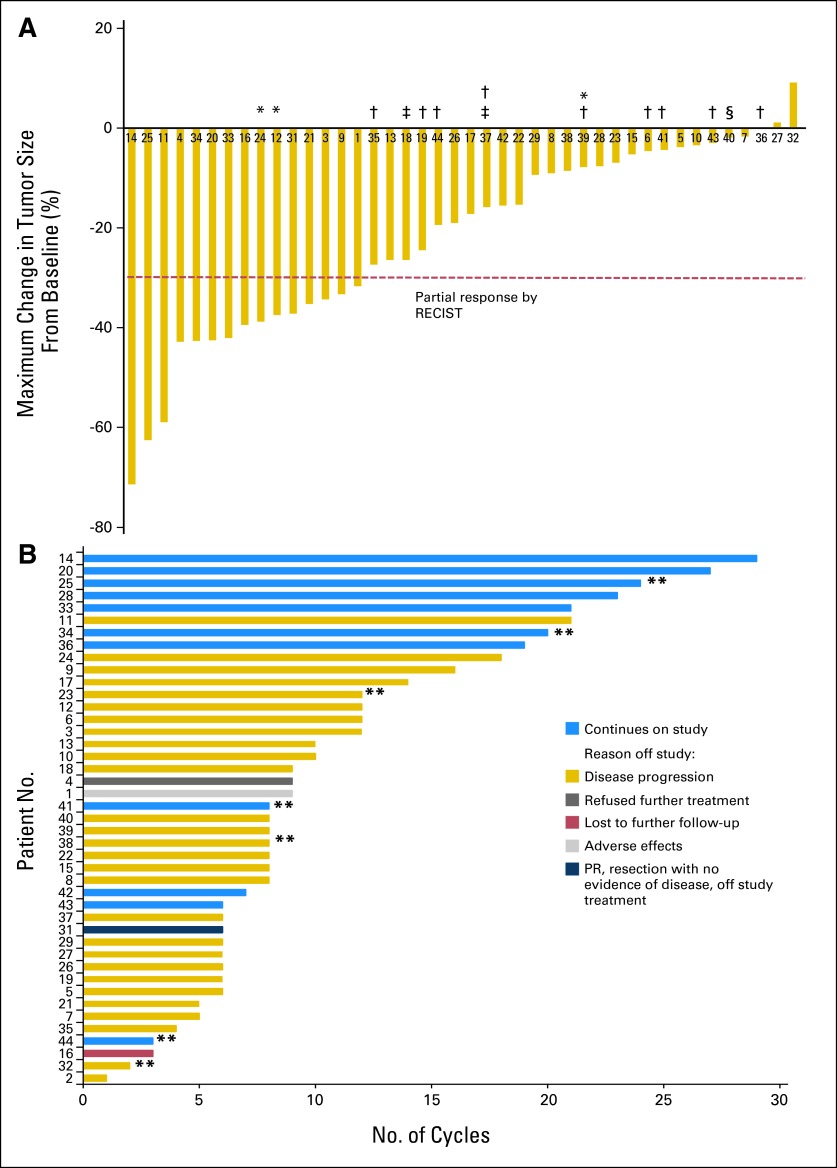
Fig 1.
Tumor response in 43 evaluable patients. (A) Maximal change in tumor size from baseline assessed according to RECIST (version 1.0), which uses 30% shrinkage in the sum of the longest diameters of target lesions as the threshold for partial response (PR; dashed line). The patient number for each patient entered onto the trial is shown below each bar. (*) Prior ARQ 197 treatment. (†) Prior sorafenib treatment. (‡) Prior sunitinib treatment. (§) Prior bevacizumab treatment. One patient died before follow-up assessment. (B) Duration on study for each evaluable patient through the data analysis date of June 30, 2012. (**) Patients who underwent paired tumor biopsies (see Fig 3).