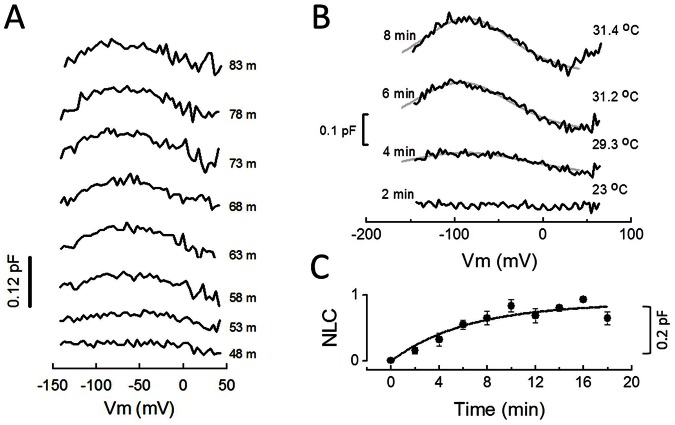
Figure 1. Prestin delivery to the cell surface can be monitored in real time by NLC measurements.
A) For initial experiments, tet-inducible HEK293 cells were incubated with 1.0 µg/ml tetracycline for 30 min at 37°C prior to whole cell recording in normal growth media plus tetracycline at room temperature (24°C). In this case, intracellular (pipette) solution was (in mM) 128 KCl, 5 MgCl2, 0.5 CaCl2, 5 EGTA, pH7.28. Cell membrane capacitance traces were recorded every 5 minutes, and the time stamp on traces indicates the time following tetracycline. Prestin NLC clearly develops over time. All traces are subtracted with the 43 minute trace. B) Synchronized transient delivery of newly synthesized prestin to the plasma membrane from TGN occurs after release of T-block. Freshly plated tet-inducible HEK293 cells on cover slips were allowed to settle for 3 hours at 37°C before incubation with 1.0 µg/ml tetracycline for 60 min at 21°C for protein synthesis. Cells were then transferred to a bath solution composed of normal growth media plus tetracycline supplemented with 4 mM 4-AP and 5 mM TEA for whole-cell patch clamp. Intracellular (pipette) solution is given in Methods. Cell membrane capacitance traces were taken every 2 minutes, and the release of temperature block was initiated by increasing the temperature of the bath solution after whole-cell configuration was established and the cell was detached using the pipette. In order to emphasize the increase over time, NLC traces are subtracted with 0 time trace. Prestin trafficking from trans-Golgi network to the plasma membrane occurs quickly. C) Normalized NLC change during transient delivery of prestin to the plasma membrane following release of T-block. NLC changes either in Peak NLC or in fitted Qmax were averaged (+/− se, n = 3–8 cells) for each time point as measured in B. Normalized NLC data were fit with a single exponential, τ being 6.4 minutes. The plateau likely reflects the depletion of membrane-bound prestin molecules from the trans-Golgi complex.